Rotating electrode in electro membrane extraction: a new and efficient methodology to increase analyte mass transfer
Sakine Asadia,
Hadi Tabani*b,
Kamal Khodaeib,
Farhad Asadianb and
Saeed Nojavan*a
aDepartment of Pure Chemistry, Faculty of Chemistry, Shahid Beheshti University, G. C., P.O. Box 19396-4716, Evin, Tehran, Iran. E-mail: s_nojavan@sbu.ac.ir; Fax: +98 21 22431661; Tel: +98 21 22431661
bDepartment of Environmental Geology, Research Institute of Applied Sciences (ACECR), Shahid Beheshti University, Tehran, Iran. E-mail: hadi_tabani@yahoo.com
First published on 20th October 2016
Abstract
Introducing a new approach to a methodology is an interesting task and represents a hot topic in the field of sample preparation. This work is the first study to introduce rotating electrode electro membrane extraction (REEME) as an efficient and inexpensive method. In this new approach to EME, the acceptor phase (AP) was agitated using a rotating electrode that was connected to a modulated speed rotator. The aim of this set up was to enhance the extraction efficiency by increasing analyte mass transfer from supported liquid membrane (SLM) to the AP and reducing the thickness of the double-layer in the inner side of the SLM. Box–Behnken design and response surface methodology were applied to find optimal experimental conditions for the extraction of a few model basic drugs including verapamil, haloperidol, and rivastigmine. Under the optimized conditions (organic solvent in the SLM: 2-ethyl hexanol, stirring rate of AP: 64 rpm, stirring rate of sample solution: 1400 rpm, potential difference: 170 V, extraction time: 16 min, acceptor phase's pH: 2.0, and donor phase's pH: 5.0), limits of detection and quantification were in the ranges of 2.0–3.0 ng mL−1 and 6.0–10.0 ng mL−1, respectively. To understand the influence of agitation of the AP on extraction efficiency, a comparative study was carried out between conventional EME and REEME methods. The results showed that, the extraction efficiency of REEME was higher than that of EME. Finally, the proposed method was successfully applied to determine concentrations of model drugs in wastewater and urine samples.
1. Introduction
During the last decade, liquid phase microextraction (LPME) has gained considerable attention due to its advantages over conventional methods of sample preparation; the advantages include versatility, high efficiency, and minimal consumption of organic solvents.1 Also, an alternative concept of LPME has been introduced based on the use of single, low-cost, disposable and porous hollow fibers made of polypropylene to support organic phase in pores of wall while holding the second aqueous phase in the lumen of the fiber.2–4 This mode of hollow fiber liquid phase microextraction (HF-LPME) that was proposed by Pedersen-Bjergaard et al.5. In this mode, porous hollow fiber was used to protect the extraction solvent. As the extraction solvent was not in direct contact with the sample solution, the samples might be stirred or vibrated vigorously without any significant loss of the extraction solvent. Micro pores of the hollow fiber would prevent large molecules (like proteins) and impurities from entering into the extraction solvent in the lumen. Moreover, the disposable hollow fiber could avoid cross-contamination. Thus, providing enhanced pre-concentration and sample clean-up capabilities, HF-LPME could be considered as a more robust and reliable alternative to other LPME methods. But, even though the driving force in HF-LPME is passive diffusion of the target analytes based on the distribution constants between the donor phase (DP) and the supported liquid membrane (SLM) and also between the SLM and the acceptor phase (AP), usually a long extraction time (30–100 min) is needed to extract the analytes.6 In order to overcome this limitation, electro membrane extraction (EME) was introduced by Pedersen-Bjergaard et al.7 in 2006. This method is originally a modification to HF-LPME; the modification introduces two platinum electrodes attached to a DC voltage, so as to electrokinetically facilitate movement of the analyte across the SLM. A power supply provides required DC potential difference (generally 1–400 V) to enhance the extraction rate of ionizable analytes from DP to AP. The two platinum electrodes are used to perform the migration of the analytes; one of these electrodes is placed into the sample while the other is placed inside the hollow fiber into the AP. Due to the use of a DC voltage to force analyte migration across the SLM, faster extractions are generally obtained as compared to the HF-LPME procedures.Up to now, a lot of developments have been reported on improving the advantages and overcoming the drawbacks of EME. For instance, extraction of polar analytes with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P < 1 is very difficult with pure solvent in the SLM. It has been found that, the addition of hydrophobic ion-pair reagents such as di-(2-ethylhexyl) phosphate (DEHP) or tri-(2-ethylhexyl) phosphate (TEHP) to SLM can improve phase transfer and electrokinetic migration of these analytes.8–10 Also recently, some papers have reported the addition of surfactants to sample solutions.11,12 Surfactants can form a hydrophobic layer around the analytes, enhancing their migration and tendency towards the SLM. Thus, the presence of surfactant in the DP can increase extraction efficiency compared to that of conventional EME.11,12 Continuing with the improvement of EME technique, the incorporation of nanoparticles into SLM was introduced. Modified SLM allows an additional adsorption/desorption process beside the electrokinetic transportation from DP to AP.13–16 Also, EME has been used in combination with other microextraction techniques such as dispersive liquid–liquid microextraction (DLLME),17,18 solid phase extraction (SPE),19 and solid-phase microextraction (SPME)20,21 to obtain hybrid procedures. These hybrid techniques provide the advantages of the two original techniques including better sample clean-up and higher enrichment factors of analytes, as compared to those of conventional EME.
P < 1 is very difficult with pure solvent in the SLM. It has been found that, the addition of hydrophobic ion-pair reagents such as di-(2-ethylhexyl) phosphate (DEHP) or tri-(2-ethylhexyl) phosphate (TEHP) to SLM can improve phase transfer and electrokinetic migration of these analytes.8–10 Also recently, some papers have reported the addition of surfactants to sample solutions.11,12 Surfactants can form a hydrophobic layer around the analytes, enhancing their migration and tendency towards the SLM. Thus, the presence of surfactant in the DP can increase extraction efficiency compared to that of conventional EME.11,12 Continuing with the improvement of EME technique, the incorporation of nanoparticles into SLM was introduced. Modified SLM allows an additional adsorption/desorption process beside the electrokinetic transportation from DP to AP.13–16 Also, EME has been used in combination with other microextraction techniques such as dispersive liquid–liquid microextraction (DLLME),17,18 solid phase extraction (SPE),19 and solid-phase microextraction (SPME)20,21 to obtain hybrid procedures. These hybrid techniques provide the advantages of the two original techniques including better sample clean-up and higher enrichment factors of analytes, as compared to those of conventional EME.
In order to improve recovery and/or enrichment, Pedersen-Bjergaard et al. introduced on-chip dynamic-EME (on-chip d-EME) and lab-on-chip systems22–24 which enabled continuous delivery of donor and acceptor solutions. This methodology allows one to perform analyses with very small volumes of sample while achieving low chemicals and reagents consumption, high extraction efficiency, and rapid extraction because of a very short diffusion path.23 Another interesting developments realized in the context of EME is the use of pulsed voltage, instead of constant voltage, in EME. Referred to as pulse EME, this method was introduced by Yamini et al.25 It provides a very stable system by decreasing the thickness of the double layer at the SLM, improving the extraction efficiency. Kuban et al.26 proposed a new concept of micro-preparative based on micro-electromembrane extractions (μ-EMEs) across free liquid membranes (FLMs). The presented concept of μ-EMEs with a single microextraction unit greatly improved variability of extraction conditions since the number of the plugs, their composition, volume and sequence in the unit could be easily altered.26–28 In an interesting study, Davarani et al. developed a virtually rotating SLM for the elimination of double layer around the hollow fiber.29 The proposed method was based on the replacement of the outer electrode by five electrodes. Five electrodes were located on the corners of a hypothetical pentagon with the SLM in the center. The voltage was applied by an electronic circuit to the electrodes in a rotatory pattern resembling virtual rotation of the SLM. The proposed method gave higher recoveries up to 50% greater than those of a conventional EME method. It also allowed the extraction to be carried out at shorter time and lower voltage.29
Overall mass transfer in EME is due to electrokinetic migration, convection, and diffusion,.30 The principal effect of stirring is to reduce the boundary layer thickness at the sample solution/SLM interface.7,30,31 Stirring of the DP enhances the diffusion of analyte by accelerating the mass transfer in the DP while reducing the thickness of Nernst's diffusion film around the DP/SLM interface. By further stirring, convection becomes dominant in the bulk sample and the boundary layer thickness is reduced, both of which are beneficial to the extraction process. It also reduces the time needed to reach equilibrium and shortens the extraction time by raising diffusion rate of the analytes from DP into AP.30,31 Although the problem of mass transfer in DP to SLM would be resolved with this arrangement, there are still double layers at the SLM/AP interface that decrease the extraction efficiency. Thus, during the extraction, analyte concentration near the inner SLM is higher than that at the middle of that, i.e. there is a concentration gradient in the lumen of hollow fiber. As such, one should stir the AP to get rid of this problem.
As of now, the reports on the effect of agitation of the DP on extraction recovery were discussed. Too small inner diameter of lumen of the hollow fiber makes its agitation a very difficult practice. Therefore, in this study, we attempted to design a system for agitation of the AP. For this purpose, a rotating electrode that was connected to a modulated speed rotator was used to stir the AP. To the best of our knowledge, it is the first report on employing a rotating electrode in EME. Verapamil (VER), haloperidol (HAL), and rivastigmine (RIV) were used, as model basic drugs, to validate applicability and efficiency of the designed REEME setup. Box–Behnken design (BBD) was used to optimize the effects of different experimental conditions on the extraction procedure. Then, the results were compared with those of conventional EME, so as to investigate the role of AP agitation during the extraction. Finally, the optimized procedure was employed to determine the model drugs in wastewater and urine samples.
2. Experimental
2.1. Reagents and materials
Used with no further purification, all model drugs, VER, HAL, and RIV (purity > 99%) were kindly supplied from Tofigh Daru Pharmaceutical Company (Tehran, Iran). All the analytical grade solvents including 2-nitrophenyl octyl ether (NPOE), methanol, acetone, acetonitrile, 2-ethyl hexanol, 1-hexanol, 1-heptanol, and 1-octanol were purchased from Merck (Darmstadt, Germany). HPLC grade water was obtained through a Milli-Q® system (Millipore, Milford, MA, USA) and was used to prepare all solutions.2.2. Standard and real sample solutions
Stock solution of each drug (1000 mg L−1) was prepared in HPLC grade water. The stock solutions were protected from light and stored for one month at 4 °C with no evidence of decomposition. All required standard solutions were daily prepared from these stock solutions and were diluted with HPLC grade water. The sample solutions had their pH values adjusted using HCl (1.0 M) and NaOH (1.0 M) solutions. Wastewater samples were obtained from Tofigh Daru Company (Tehran, Iran) and urine samples were obtained from the Clinic of Taleghani Hospital (Tehran, Iran). The samples were stored at −4 °C, thawed and shaken before extraction.2.3. Chromatographic conditions
Separation, identification and quantification were carried out on an Agilent Technologies 1200 series system consisting of a solvent degasser (G1322A), a quaternary pump (G1311A), and a manual injection valve (G1328B) equipped with a 20 μL injection loop and a variable wavelength UV detector (G1314B). Separations were carried out on an ODS-3 MZ analytical column (250 mm × 4.6 mm, 5 μm) (Grace Vydac Inc., Worms., Germany). The chromatographic separation of drugs was performed with a mobile phase consisting of 10 mM phosphate buffer, with its pH adjusted to 3.0, and acetonitrile delivered at 1.2 mL min−1. The gradient program was as follows: starting with 40% acetonitrile, then increasing to 80% in 10 min, thereafter restored to 40% in 4 min. The detector wavelength was set to 210 nm for all drugs. ChemStation Software (Agilent Technologies) was used for acquiring and processing the data.2.4. REEME equipment and extraction procedure
Fig. 1 shows the equipment used to implement the proposed extraction procedure. The used DC power supply was a PV-300 (Mobtaker Aryaei J., Zanjan, Iran) with programmable voltage in the range of 0–600 V, providing currents in the range of 0–0.5 A. The electrodes used in this work were platinum wires with a diameter of 0.2 mm; those were purchased from Pars Pelatine (Tehran, Iran). As shown in Fig. 1, the tip of one of the electrodes (cathode) was connected to the shaft of an electric motor, serving as the rotating electrode. A modulated speed rotator (Durham, NC. 27705. USA) was used to control rotation speed of the motor. The porous hollow fiber used to immobilize the SLM and provide housing for the AP was a PP Q3/2 polypropylene hollow fiber (Membrana, Wuppertal, Germany) with an internal diameter of 1.2 mm, a wall thickness of 200 μm, and pores of 0.2 μm in diameter. It was cut into 3.2 cm segments, cleaned in acetone and dried prior to use. Stirring of the solutions was carried out by a Heidolph MR 3001 K magnetic stirrer (Schwabach, Germany) equipped with 1.5 mm × 8 mm magnetic bars.The sample solution (6.5 mL at pH = 5.0) containing target drugs was transferred into the sample vial. To have the pores of hollow fiber walls impregnated by the organic solvent, the hollow fiber was dipped into the organic solvent (2-ethyl hexanol) for 30 s. 30 μL of the aqueous acceptor solution (pH = 2.0) was introduced, by a microsyringe, into the lumen of the hollow fiber. The lower end of the hollow fiber was then sealed using a pair of hot flat-tip pliers. The upper end of the fiber was connected to a medical needle tip, serving as a guiding tube, which was inserted through the rubber cap of the vial. The rotating electrode (cathode) was introduced into the lumen of the fiber, with the other electrode inserted into the sample solution. The AP and sample solution were stirred by the rotating electrode and magnetic stirrer at 64 rpm and 1400 rpm, respectively. A 170 V voltage was powered on and the extraction was performed for 16 min. Once the extraction was completed, a microsyringe was used to collect the AP and inject it into the HPLC for further analysis.
2.5. Data analysis and statistical methods
In order to obtain optimal conditions and investigate the interaction of variables, a BBD was employed. The experimental design matrix and data analysis were performed by the Statgraphics Plus Package (version 5.1; Statistical Graphics, Manugistics, USA).323. Results and discussion
3.1. Optimization strategy
The aim of the experimental design was to determine the main variables imposing the largest effects on efficiency of model drugs. The preliminary experiments were carried out to select the variables of the greatest influences on the extraction efficiency of analytes. Initially, the influences of type of organic solvent and pH of the donor and acceptor phases on extraction efficiency were evaluated using one-variable-at-a-time (OVAT) methodology. Then, important variables (extraction time, applied voltage and stirring rate of the AP) were selected to generate a BBD in order to build a predictive model for the response. Finally, the response surface plots based on the defined models were chosen to find the optimal experimental conditions leading to the maximum response. As is reported in Table 1, levels of the selected factors were chosen based on preliminary experiments.| Variable | Key | Level | ||
|---|---|---|---|---|
| Lower | Central | Upper | ||
| Voltage (volt) | A | 50 | 125 | 200 |
| Extraction time (min) | B | 2 | 10 | 18 |
| Rotation (rpm) | C | 30 | 75 | 120 |
According to previous studies, the formation of intense whirlpool in the DP and bubbles around the hollow fiber at stirring rates of higher than 1400 rpm significantly decrease the extractability.33,34 Thus, a maximum stirring rate (1400 rpm) was set in all the experiments. Also, salt addition into the sample leads to increase the competition among target analytes and interfering ions, which in turn decreases the flux of target analytes across the SLM. In addition, by increasing the concentrations of ions in the DP, the number of ions across the SLM are increased, consequently leading to an increase in the friction between ions and the organic solvent, generation of excessive heat (joule heating), and finally, instability of the SLM.35,36 Thus, no salt was added to the sample solution for the rest of the experiments. All experiments were conducted in solutions containing 200 ng mL−1 of each model drug.
 | (1) |
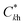 is the concentration of the anionic substance in the DP, Cio is the concentration of the cationic substance in the AP, and
is the concentration of the anionic substance in the DP, Cio is the concentration of the cationic substance in the AP, and  is the concentration of the anionic substance in the AP. Thus, the ratio of total ionic concentration in the DP to that in the AP represents the flux over the membrane.37 As predicted by the theoretical model,38 maximum response can be obtained at the minimum value of χ. Investigating the effect of this parameter, pH of the AP and pH of the DP were changed in the ranges of 1.0–4.0 and 3.0–7.0, respectively. In EME, pH of the DP should be adjusted at a value to convert the analytes in their ionized form providing the possibility of electrokinetic migration by applying an electrical driving force. Thus, in order to extract basic drugs, the DP should be adequately acidified to keep the analytes with positive charges. The results showed that, extraction efficiency of the drugs increases significantly with increasing pH of the DP up to 5.0, because of decreasing the value of χ (Fig. 3A). When pH of the DP was further increased from 5.0 to 7.0, the extraction efficiency of each drug was slightly decreased. This could be attributed to high pH of the DP which would reduce the ionization efficiency of the drugs. Thus, a pH of 5.0 was taken as the optimal pH of the DP for the rest of experiments.
is the concentration of the anionic substance in the AP. Thus, the ratio of total ionic concentration in the DP to that in the AP represents the flux over the membrane.37 As predicted by the theoretical model,38 maximum response can be obtained at the minimum value of χ. Investigating the effect of this parameter, pH of the AP and pH of the DP were changed in the ranges of 1.0–4.0 and 3.0–7.0, respectively. In EME, pH of the DP should be adjusted at a value to convert the analytes in their ionized form providing the possibility of electrokinetic migration by applying an electrical driving force. Thus, in order to extract basic drugs, the DP should be adequately acidified to keep the analytes with positive charges. The results showed that, extraction efficiency of the drugs increases significantly with increasing pH of the DP up to 5.0, because of decreasing the value of χ (Fig. 3A). When pH of the DP was further increased from 5.0 to 7.0, the extraction efficiency of each drug was slightly decreased. This could be attributed to high pH of the DP which would reduce the ionization efficiency of the drugs. Thus, a pH of 5.0 was taken as the optimal pH of the DP for the rest of experiments.
In the AP, by keeping the pH of the DP constant at 5.0, the highest extraction efficiency was obtained (Fig. 3B). The extractability of the analytes decreased with increasing pH of the AP. The increasing in χ under these conditions represented an important reason for the decreasing in extractability of the model basic drugs. Also, there were some limitations regarding very low pH values of the AP; the limitations included increased risk of bubble formation and punctuation in the AP volume due to increased electrolysis reactions on the electrode surface. For the rest of the present work, a pH value of 2.0 was utilized as optimal pH of the AP.
One of the important parameters for evaluating the model is adjusted R-squared (Radj2). This parameter can be considered as a measure of the amount of variation around the mean explained by the model adjusted for the number of terms in the model.41 In addition, predicted R-square (Rpred2) which is a measure of the amount of variation in new data explained by the model can be applied for the evaluation of the model. In this study, the values of R2 and Radj2 were calculated using backward mode of multiple linear regression model to exclude non-significant effects from the model and thus avoid over-fitting. The Rpred2 and Radj2 were 0.98 and 0.94, respectively, indicating the adequacy of the obtained equation for correlating the experimental results.
Analysis of variance (ANOVA) was used to evaluate the obtained data. The main effects and their interactions were visualized by a Pareto chart (Fig. 4). In the Pareto chart, the bar lengths are proportional to the absolute value of the estimated main effects. The chart also includes a vertical line corresponding to 95% confidence interval. An effect exceeding this reference line may be considered significant with regard to the response. This chart implies that, all the three factors and the interactions between some of them were of statistically significant effects at p < 0.05. The model was expressed as the following equation:
| Normalized peak area = −106.008 + 0.698611A + 10.9159B + 1.43477C − 0.00185556A2 + 0.00358333AB − 0.0018963AC − 0.341602B2 − 0.00548611BC − 0.0079321C2 |
For the graphical interpretation of the interactions to obtain the optimum conditions, the use of response surface methodology (RSM) on the model is highly recommended.42 In this methodology, each time, the response is plotted against two variables within the experimental range, with the other variables kept constant at their central levels. It also shows two-dimensional (2D) contour plots based on the model equation, demonstrating the interaction between independent variables; this assists in determining the optimal operating conditions. The RSM for extraction efficiency of the drugs are shown in Fig. 5A–C. Based on the analysis and presented plots in Fig. 5A, it can be observed that the normalized peak area is increased with increasing the voltage and extraction time and declined thereafter. In EME, mass transfer is a time-dependent process and the flux of analyte is affected by the magnitude of the applied potential. As a result, both the extraction time and the applied voltage are important parameters affecting extraction efficiency. Also, extraction time and the applied voltage affect the extraction efficiency of EME method concurrently,43 i.e. an increase in extraction time limits the voltage and vice versa. Consequently, these two parameters were studied simultaneously to investigate their interactions. It was demonstrated that the normalized peak area for the drugs increased by increasing the applied voltage and extraction time up to 170 V and 16 min, respectively. Further increase in voltage leads to a decrease in response due to mass transfer resistance because of the built-up of a boundary layer of ions at the interfaces on both sides of the SLM, an increase in current level and bobble formation. Also for longer extraction times, the organic solvent in the SLM was partly dissolved due to its contact with aqueous solutions on both sides of the hollow fiber, so that it might not be able to fully separate two aqueous phases. Similar behavior was previously described by Balchen et al.36 Therefore, for the rest of experiments, an electrical potential of 170 V was applied for 16 min.
 | ||
| Fig. 5 RSM and contour plots obtained by plotting of (A) voltage vs. extraction time, (B) voltage vs. stirring rate of the AP, and (C) extraction time vs. stirring rate of the AP, using the BBD. | ||
Also, the RSM and 2D contour plots were applied to analyze the effect of stirring of the AP on the response (Fig. 5B and C). As can be seen from these figures, the extraction efficiency exhibited an initial increase with the increase in rotating rate up to 64 rpm, followed by a descent with further increase in rotating rate. During the extraction, concentration of drugs near the inner side of the SLM is higher than those at middle of it, developing a concentration gradient in the lumen of HF. Agitation of the AP would increase the extraction efficiency by increasing the mass transfer and reducing the double-layer thickness at the inner side of the SLM. Thus, a rotating rate of 64 rpm was considered for the AP agitation.
3.2. Comparison of the REEME with conventional EME at different extraction times
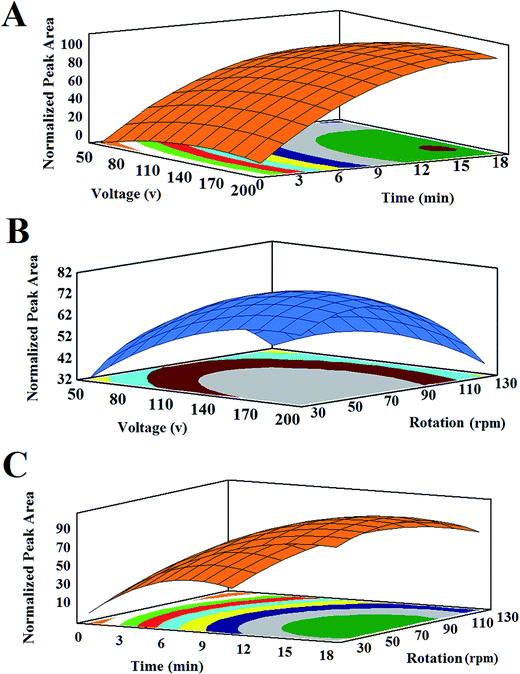
In order to investigate the influence of the AP agitation on the extraction efficiency, a comparative study was carried out between EME and REEME at different extraction times (Fig. 6). In this study, aiming at showing the ability and performance of the methods and undertake a better comparison of the results, extractions using both methods were done under the same set of conditions (organic solvent in the SLM: 2-ethyl hexanol, stirring rate of sample solution: 1400 rpm, potential difference: 170 V, acceptor phase pH: 2.0, and donor phase pH: 5.0). Of course for REEME method, the AP was stirred at 64 rpm. As can be seen from Fig. 6, in short extraction time (t = 2 min), extraction efficiencies were almost the same for both methods (compare corresponding peak areas to each drug when using either of the two methods). But in longer extraction times, REEME showed much better results than those of the conventional EME. This could be attributed to the fact that, in short extraction times (t < 2 min), near inner side the SLM is not saturated with analytes, so as no concentration gradient develops in the lumen of the hollow fiber. But with long extraction times, there would be a concentration gradient in the AP, so that the concentration of analyte near inner side of the SLM is higher than that at the middle of it. Thus, stirring of the AP can increase the extraction efficiency by decreasing the thickness of double layer at SLM/AP interface and improves extractability by eliminating this mass transfer barrier.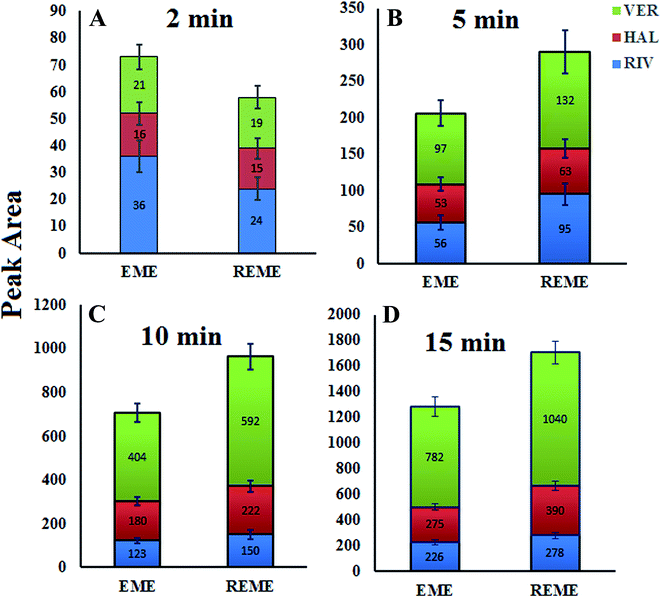 | ||
| Fig. 6 Extraction results obtained from EME and REEME at (A) 2 min, (B) 5 min, (C) 10 min, and (D) 15 min as extraction times. | ||
3.3. Validation of the method
Figures of merit of the proposed method were investigated under optimized conditions to evaluate its applicability for quantification model basic drugs. Some analytical features such as limit of detection (LOD), limit of quantification (LOQ), linearity, correlation coefficients (r), precision, and recovery (R) are shown in Table 2. The LOD for each compound in water sample was determined at a concentration where the signal to noise ratio was equal to 3. Acceptable LOD values were obtained within the range of 2.0–3.0 ng mL−1. The precision of the proposed method, was evaluated by extracting four independent samples spiked at 50 ng mL−1 with each drug and it was found to be in the range of 5.9–10.5% (Table 2).| Drug | LOQa | LODa | Linearitya | r | EF | Recoveryb | RSD%c |
|---|---|---|---|---|---|---|---|
| a Concentration is based on ng mL−1.b Recovery was obtained for 50 ng mL−1 of each drug (n = 3).c RSDs% were obtained by four replicate measurements for 50 ng mL−1 of each drug. | |||||||
| VER | 6 | 2 | 6–500 | 0.993 | 86 | 43 | 7.7 |
| HAL | 6 | 2 | 6–500 | 0.995 | 88 | 44 | 10.5 |
| RIV | 6 | 3 | 10–500 | 0.995 | 104 | 52 | 5.9 |
The recovery (R) was defined as the percentage of the number of moles of the analyte adsorbed onto the sorbent (nf) to those originally present in the sample solution (ni).
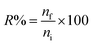 | (2) |
Relative recovery (RR) was acquired from the following equation:
 | (3) |
3.4. Analysis of real samples
The applicability of the extraction method to real samples was examined by extraction and determination of VER, HAL, and RIV in urine and wastewater samples. In order to reduce the matrix effect, the urine samples were diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio using double distilled water and then spiked with target drugs; the wastewater samples were used without any dilution. Finally, the extraction was carried out under the most appropriate conditions and the results of three replicated analyses of each sample using the proposed method are shown in Table 3. The results showed that the analytes were not detected in any of the analyzed real samples. To investigate the matrix effects, these samples were spiked with each of model drugs at 30 ng mL−1 and 80 ng mL−1 levels and their relative recoveries were determined to be in the ranges of 78–105% and 81–105%, respectively (Table 3). These high relative recoveries indicated a negligible matrix effect on REEME efficiency in these samples. Corresponding chromatograms to analyte extraction from the wastewater and urine samples are shown in Fig. 7.
3 ratio using double distilled water and then spiked with target drugs; the wastewater samples were used without any dilution. Finally, the extraction was carried out under the most appropriate conditions and the results of three replicated analyses of each sample using the proposed method are shown in Table 3. The results showed that the analytes were not detected in any of the analyzed real samples. To investigate the matrix effects, these samples were spiked with each of model drugs at 30 ng mL−1 and 80 ng mL−1 levels and their relative recoveries were determined to be in the ranges of 78–105% and 81–105%, respectively (Table 3). These high relative recoveries indicated a negligible matrix effect on REEME efficiency in these samples. Corresponding chromatograms to analyte extraction from the wastewater and urine samples are shown in Fig. 7.
| Sample | RIV | HAL | VER | |
|---|---|---|---|---|
| a Not detected. | ||||
| Urine 1 | Initial concentration (ng mL−1) | nda | nd | nd |
| Spikes concentration (ng mL−1) | 30 | 30 | 30 | |
| RR%a | 87 | 104 | 96 | |
| RSD% (n = 3) | 2.6 | 5.3 | 1.3 | |
| Initial concentration (ng mL−1) | nd | nd | nd | |
| Spikes concentration (ng mL−1) | 80 | 80 | 80 | |
| RR%a | 81 | 102 | 94 | |
| RSD% (n = 3) | 6.8 | 7.1 | 11 | |
| Urine 2 | Initial concentration (ng mL−1) | nd | nd | nd |
| Spikes concentration (ng mL−1) | 30 | 30 | 30 | |
| RR%a | 78 | 103 | 101 | |
| RSD% (n = 3) | 7.9 | 12.3 | 8.3 | |
| Initial concentration (ng mL−1) | nd | nd | nd | |
| Spikes concentration (ng mL−1) | 80 | 80 | 80 | |
| RR%a | 62 | 103 | 99 | |
| RSD% (n = 3) | 6.5 | 7.1 | 9.1 | |
| Wastewater | Initial concentration (ng mL−1) | nd | nd | nd |
| Spikes concentration (ng mL−1) | 30 | 30 | 30 | |
| RR%a | 85 | 105 | 105 | |
| RSD% (n = 3) | 5.4 | 11.5 | 14 | |
| Initial concentration (ng mL−1) | nd | nd | nd | |
| Spikes concentration (ng mL−1) | 80 | 80 | 80 | |
| RR%a | 82 | 105 | 103 | |
| RSD% (n = 3) | 1.6 | 4.4 | 6.7 | |
4. Conclusions
A new EME approach was introduced, for the first time, for the extraction of basic drugs from different matrices. Unlike conventional EME methods, in the REEME technique the AP was agitated using a rotating electrode for which a new setup was designed. In this study, the effect of stirring rate of the AP was investigated to figure out the role of agitation in increasing mass transfer and reducing the thickness of Nernst's diffusion film around the SLM/AP interface. RSM-BBD was successfully employed to optimize and study the individual and interactive effect of variables (voltage, extraction time, and rotating rate of the AP) on REEME process. In comparison with conventional EME, the proposed method presents better analytical characteristics. Moreover, the main advantages of the proposed method are significantly decreased extraction time coupled with increased extraction efficiency compared to conventional EME. The developed method was employed to determine VER, HAL, and RIV concentrations as model analytes in spiked urine samples, so as to demonstrate the method capacity for real cases. This technique also demonstrated a high degree of sample clean-up. As hollow fiber is disposable, single use of the hollow fiber reduces the risk of cross-contamination. Regarding the mentioned points above and better figure of merit provided by the proposed REEME, this technique may be introduced as a novel and efficient technique for the extraction of various analytes from different matrices.Acknowledgements
Financial support from the Research Affairs of Shahid Beheshti University is gratefully acknowledged.References
- A. R. Fakhari, H. Tabani and S. Nojavan, Immersed single-drop microextraction combined with gas chromatography for the determination of sufentanil and alfentanil in urine and wastewater samples, Anal. Methods, 2011, 3, 951–956 RSC
.
- C. Basheer, H. K. Lee and J. P. Obbard, Determination of organochlorine pesticides in seawater using liquid-phase hollow fibre membrane microextraction and gas chromatography–mass spectrometry, J. Chromatogr. A, 2002, 968, 191–199 CrossRef CAS PubMed
.
- S. Pedersen-Bjergaard and K. E. Rasmussen, Liquid–liquid–liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis, Anal. Chem., 1999, 71, 2650–2656 CrossRef CAS PubMed
.
- A. R. Fakhari, H. Tabani and S. Nojavan, Miniaturized hollow fibre assisted liquid-phase microextraction and gas chromatography for determination of trace concentration of sufentanil and alfentanil in biological samples, Drug Test. Anal., 2013, 5, 589–595 CrossRef CAS PubMed
.
- S. Pedersen-Bjergaard and K. E. Rasmussen, Liquid–liquid–liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis, Anal. Chem., 1999, 71, 2650 CrossRef CAS PubMed
.
- K. E. Rasmussen and S. Pedersen-Bjergaard, Developments in hollow fibre-based, liquid-phase microextraction, Trends Anal. Chem., 2004, 23, 1–10 CrossRef CAS
.
- S. Pedersen-Bjergaard and K. E. Rasmussen, Electrokinetic migration across artificial liquid membranes – new concept for rapid sample preparation of biological fluids, J. Chromatogr. A, 2006, 1109, 183–190 CrossRef CAS PubMed
.
- A. Gjelstad, K. E. Rasmussen and S. Pedersen-Bjergaard, Electrokinetic migration across artificial liquid membranes tuning the membrane chemistry to different types of drug substances, J. Chromatogr. A, 2006, 1124, 29–34 CrossRef CAS PubMed
.
- H. Tabani, A. R. Fakhari and A. Shahsavani, Simultaneous determination of acidic and basic drugs using dual hollow fiber electromembrane extraction combined with CE, Electrophoresis, 2013, 34, 269–276 CrossRef CAS PubMed
.
- M. H. Koruni, H. Tabani, H. Gharari and A. R. Fakhari, An all-in-one electro-membrane extraction: development of an electro-membrane extraction method for the simultaneous extraction of acidic and basic drugs with a wide range of polarities, J. Chromatogr. A, 2014, 1361, 95–99 CrossRef CAS PubMed
.
- P. Zahedi, S. S. H. Davarani, H. R. Moazami and S. Nojavan, Surfactant assisted pulsed two-phase electromembrane extraction followed by GC analysis for quantification of basic drugs in biological samples, J. Pharm. Biomed. Anal., 2016, 117, 485–491 CrossRef CAS PubMed
.
- K. S. Hasheminasab and A. R. Fakhari, Application of nonionic surfactant as a new method for the enhancement of electromembrane extraction performance for determination of basic drugs in biological samples, J. Chromatogr. A, 2015, 1378, 1–7 CrossRef CAS PubMed
.
- K. S. Hasheminasab, A. R. Fakhari, A. Shahsavani and H. Ahmar, A new method for the enhancement of electromembrane extraction efficiency using carbon nanotube reinforced hollow fiber for the determination of acidic drugs in spiked plasma, urine, breast milk and wastewater samples, J. Chromatogr. A, 2013, 1285, 1–6 CrossRef CAS PubMed
.
- A. Atarodi, M. Chamsaz, A. Z. Moghaddam and H. Tabani, Introduction of high nitrogen doped graphene as a new cationic carrier in electromembrane extraction, Electrophoresis, 2016, 37, 1191–1200 CrossRef CAS PubMed
.
- M. Ramos-Payán, R. Fernandez-Torres, J. L. Perez-Bernal, M. Callejón-Mochón and M. A. Bello-López, A novel approach for electromembrane extraction based on the use of silver nanometallic-decorated hollow fiber, Anal. Chim. Acta, 2014, 849, 7–11 CrossRef PubMed
.
- C. R. Hidalgo, M. Ramos-Payán, J. A. Ocaña-González, M. J. Martín-Valero and M. Á. Bello-López, Agar films containing silver nanoparticles as new supports for electromembrane extraction, Anal. Bioanal. Chem., 2015, 407, 1519–1525 CrossRef CAS PubMed
.
- L. Guo and H. K. Lee, Electro membrane extraction followed by low-density solvent based ultrasound-assisted emulsification microextraction combined with derivatization for determining chlorophenols and analysis by gas chromatography–mass spectrometry, J. Chromatogr., A, 2012, 1243, 14–22 CrossRef CAS PubMed
.
- S. Seidi, Y. Yamini and M. Rezazadeh, Combination of electromembrane extraction with dispersive liquid–liquid microextraction followed by gas chromatographic analysis as a fast and sensitive technique for determination of tricyclic antidepressants, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 913–914, 138–146 CrossRef CAS PubMed
.
- H. Tabani, A. R. Fakhari, A. Shahsavani, M. Behbahani, M. Salarian, A. Bagheri and S. Nojavan, Combination of graphene oxide-based solid phase extraction and electro membrane extraction for the preconcentration of chlorophenoxy acid herbicides in environmental samples, J. Chromatogr., A, 2013, 1300, 227–235 CrossRef CAS PubMed
.
- M. Rezazadeh, Y. Yamini, S. Seidi and B. Ebrahimpour, Electromembrane surrounded solid phase microextraction: a novel approach for efficient extraction from complicated matrices, J. Chromatogr. A, 2013, 1280, 16–22 CrossRef CAS PubMed
.
- E. Mohammadkhani, Y. Yamini, M. Rezazadeh and S. Seidi, Electromembrane surrounded solid phase microextraction using electrochemically synthesized nanostructured polypyrrole fiber, J. Chromatogr. A, 2016, 1443, 75–82 CrossRef CAS PubMed
.
- N. J. Petersen, H. Jensen, S. H. Hansen, K. E. Rasmussen and S. Pedersen-Bjergaard, Drop-to-drop microextraction across a supported liquid membrane by an electrical field under stagnant conditions, J. Chromatogr. A, 2009, 1216, 1496–1502 CrossRef CAS PubMed
.
- N. J. Petersen, H. Jensen, S. H. Hansen, S. T. Foss, D. Snakenborg and S. Pedersen-Bjergaard, On-chip electromembrane extraction, Microfluid. Nanofluid., 2010, 9, 881 CrossRef CAS
.
- N. Drouin, S. Rudaz and J. Schappler, Dynamic-electromembrane extraction (d-EME): a new technical development for the extraction of neuropeptides, Anal. Chem., 2016, 88, 5308–5315 CrossRef CAS PubMed
.
- M. Rezazadeh, Y. Yamini, S. Seidi and A. Esrafili, Pulsed electromembrane extraction: a new concept of electrically enhanced extraction, J. Chromatogr. A, 2012, 1262, 214–218 CrossRef CAS PubMed
.
- P. Kuban and P. Bocek, Micro-electromembrane extraction across free liquid membranes. Instrumentation and basic principles, J. Chromatogr. A, 2014, 1346, 25–33 CrossRef CAS PubMed
.
- P. Kuban and P. Bocek, Simultaneous micro-electromembrane extractions of anions and cations using multiple free liquid membranes and acceptor solutions, Anal. Chim. Acta, 2016, 908, 113–120 CrossRef CAS PubMed
.
- P. Kuban and P. Bocek, Preconcentration in micro-electromembrane extraction across free liquid membranes, Anal. Chim. Acta, 2014, 848, 43–50 CrossRef CAS PubMed
.
- S. S. H. Davarani, H. R. Moazami, E. Memarian and S. Nojavan, Electromembrane extraction through a virtually rotating supported liquid membrane, Electrophoresis, 2016, 37, 339–346 CrossRef PubMed
.
- A. Gjelstad, T. M. Andersen, K. E. Rasmussen and S. Pedersen-Bjergaard, Microextraction across supported liquid membranes forced by pH gradients and electrical fields, J. Chromatogr. A, 2007, 1157, 38–45 CrossRef CAS PubMed
.
- M. Balchen, A. Gjelstad, K. E. Rasmussen and S. Pedersen-Bjergaard, Electrokinetic migration of acidic drugs across a supported liquid membrane, J. Chromatogr. A, 2007, 1152, 220–225 CrossRef CAS PubMed
.
- N. Jimenez, M. Vinas, J. M. Bayona, J. Albaiges and A. M. Solanas, The Prestige oil spill: bacterial community dynamics during a field biostimulation assay, Appl. Microbiol. Biotechnol., 2007, 77, 935–945 CrossRef CAS PubMed
.
- A. R. Fakhari, H. Tabani, S. Nojavan and H. Abedi, Electromembrane extraction combined with cyclodextrin-modified capillary electrophoresis for the quantification of trimipramine enantiomers, Electrophoresis, 2012, 33, 506–515 CrossRef CAS PubMed
.
- A. R. Fakhari, H. Tabani, H. Behdad, S. Nojavan and M. Taghizadeh, Electrically-enhanced microextraction combined with maltodextrin-modified capillary electrophoresis for quantification of tolterodine enantiomers in biological samples, Microchem. J., 2013, 106, 186–193 CrossRef CAS
.
- J. Lee, F. Khalilian, H. Bagheri and H. K. Lee, Optimization of some experimental parameters in the electro membrane extraction of chlorophenols from sea water, J. Chromatogr. A, 2009, 1216, 7687–7693 CrossRef CAS PubMed
.
- M. Balchen, T. G. Halvorsen, L. Reubsaet and S. Pedersen-Bjergaard, Rapid isolation of angiotensin peptides from plasma by electromembrane
extraction, J. Chromatogr. A, 2009, 1216, 6900–6905 CrossRef CAS PubMed
.
- A. Gjelstad, K. E. Rasmussen and S. Pedersen-Bjergaard, Simulation of flux during electro-membrane extraction based on the Nernst–Planck equation, J. Chromatogr. A, 2007, 1174, 104–111 CrossRef CAS PubMed
.
- T. M. Middelthon-Bruer, A. Gjelstad, K. E. Rasmussen and S. Pedersen-Bjergaard, Parameters affecting electromembrane extraction of basic drugs, J. Sep. Sci., 2008, 31, 753–759 CrossRef CAS PubMed
.
- B. Kiran, A. Kaushik and C. P. Kaushik, Response surface methodological approach for optimizing removal of Cr(VI) from aqueous solution using immobilized cyanobacterium, Chem. Eng. J., 2007, 126, 147–153 CrossRef CAS
.
- K. Ravikumar, K. Pakshirajan, T. Swaminathan and K. Balu, Kinetic electro membrane extraction under stagnant conditions-Fast isolation of drugs from untreated human plasma, Chem. Eng. J., 2005, 105, 131–138 CrossRef CAS
.
- A. M. Joglekar and A. T. May, Product excellence through design of experiments, Cereal Foods World, 1987, 32, 857–868 Search PubMed
.
- D. C. Montgomery, Design and Analysis of Experiments, Wiley, New York, 2001 Search PubMed
.
- S. Seidi, Y. Yamini, A. Heydari, M. Moradi, A. Esrafili and M. Rezazadeh, Determination of the baine in water samples, biological fluids, poppy capsule, and narcotic drugs, using electromembrane extraction followed by high-performance liquid chromatography analysis, Anal. Chim. Acta, 2011, 701, 181–188 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |





