Engineering functional alginate beads for encapsulation of Pickering emulsions stabilized by colloidal particles†
Hongshan Liangac,
Bin Zhoud,
Jing Liac,
Yun Heac,
Yaqiong Peiac and
Bin Li*abc
aCollege of Food Science and Technology, Huazhong Agricultural University, Wuhan 430070, China. E-mail: libinfood@mail.hzau.edu.cn; Fax: +86-27-87282966; Tel: +86-27-63730040
bHubei Collaborative Innovation Centre for Industrial Fermentation, Hubei University of Technology, Wuhan 430068, China
cKey Laboratory of Environment Correlative Dietology, Huazhong Agricultural University, Ministry of Education, China
dCollege of Food Science and Technology, Shanghai Ocean University, LinGang New City, Shanghai 201306, China
First published on 19th October 2016
Abstract
Pickering emulsions are widely used as delivery systems in food, cosmetics, and pharmaceutical industries for the encapsulation and sustained release of hydrophilic compounds. The solid colloidal particles stabilized in oil–water interface can prevent the oil droplets from undesired coalescence and endow additional functionality. In order to impact further protection and pH-responsive deliver of the encapsulated component, we used two kinds of mechanisms. For internal interaction, the excess Ca2+ on the surface of zein–tannic acid (TA)/Ca2+ nanoparticles (NPs) interacted with alginate added to form a second layer. In the case of external gelation, a cross-linking agent (Ca2+) was dissolved in the aqueous gelling bath outside. When added the mixture of the emulsions to Ca2+ solution, alginate–Ca beads were formed to encapsulate the emulsions and further impact the pH-responsive deliver of VD3 in stomach and rapid release of it in small intestine.
1. Introduction
Oil-in-water (O/W) emulsions play an important role in a wide range of industrial processes and commercial products.1 Among them, Pickering emulsions, wherein solid colloidal particles instead of molecular surfactants or amphiphilic polymers adsorb at the oil/water interface, are known to display long-term stability against coalescence.2 Thereby, this kind of emulsions can provide variety of promising applications for texture modification, calorie and fat reduction, as well as bioactive compound delivery.3–5 Compared with conventional emulsions, solid colloidal particles stabilized in oil–water interface can endow additional functionality including the response to a trigger, forming bilayer shell and the like.2,6,7Different types of solid particles,8–11 such as silica,12–14 calcium carbonate (CaCO3) particles,15 titanium dioxide (TiO2) particles,16 polystyrene lattices17 and gold nanoparticles10 have been used as emulsifiers to stabilize Pickering emulsions. However, in regard to their suitability for food applications and environmental problems, the direct application of such particulate emulsifiers is still very limited.18 Although promising emulsifiers based on protein particles,19 polysaccharide particles20,21 and protein–polysaccharide complex22 have emerged, studies on food-grade Pickering emulsions for food applications are still in a limited number. Thus, the development of effective colloidal particles based on food-grade ingredients to serve as Pickering emulsion stabilizers is still a significant challenge in the food emulsion area.23
As an alcohol-soluble protein obtained from corn, zein contains sharply defined hydrophobic and hydrophilic domains and is capable of forming self-assembled NPs to stabilize Pickering emulsions.24–26 Therefore, zein-based particles possess great potential to be used as food-grade Pickering emulsion stabilizers. de Folter and co-workers have first used zein colloidal particles as effective particle-stabilizers of oil-in-water Pickering emulsions.27 Then they demonstrated the behavior of zein particles in the formation of stable Pickering emulsions as a function of particle concentration, pH and ionic strength. The followers devoted to further increasing the stabilization of oil–water interfaces with zein complex colloidal particles. Gao et al. prepared the complexation of zein particles and surfactant sodium stearate which increased the adsorption and accumulation of zein particles at oil–water interface.4 The resultant Pickering emulsions stabilized by the complexation exhibited superior stability against both coalescence and creaming. Then in order to reduce the lipid oxidation of emulsified oil, Wang et al. fabricated zein/chitosan complex particles loaded with antioxidant curcumin, and further prepared antioxidant Pickering emulsions, giving rise to the emulsions with favorable oxidative stability.22 Food-grade colloidal complexes based on zein and tannic acid were prepared and successfully used to stable Pickering emulsion gels.25
As a kind of natural polyphenol and a food-grade material, tannic acid (TA), which contains numerous terminal hydroxyl groups, has unique structural properties.28,29 The dominant constituents facilitate interactions with a variety of materials including proteins,30 polysaccharides31 and synthetic polymers32,33 via multiple reaction pathways, such as electrostatic, hydrogen bonding, and hydrophobic interactions.34 Furthermore, the strong metal chelation ability and materials surface binding affinity make it a perfect material to act as a polydentate ligand for metal ion coordination.35,36 In our previous work, TA has been well recognized as a promising material to self-assembly of coordination bonding architecture with metal ions on zein NPs.31,37 In addition, the excess metal ions on the surface of NPs could further form connections with other materials. The newly formed layer may achieve one or more desirable effects including protection from evaporation or oxidation of the loaded compound, and controlled-release applications.
Alginate is a characteristic example of natural polyelectrolytes undergoing chain–chain association and forming hydrogels upon addition of divalent cations (e.g., Ca2+).38,39 Gelation is controlled by chelation between the carboxyl groups of a-L-guluronic acid in chains of alginate with calcium.40–42 Alginate hydrogels have been extensively investigated due to their biocompatibility and pH-responsive characteristics which can provide a controlled release of encapsulated component.43–45
In this study, we first formed zein–TA/Ca2+ NPs, and consequently use this kind of particles to prepare stable Pickering emulsions for encapsulation of an unstable nutrient, VD3. In order to induce the gelation of the droplets, we mainly employed two kinds of mechanisms, namely internal interaction and external gelation.46 For internal interaction, the excess Ca2+ on the surface of NPs will interact with alginate added to form a second layer. In the case of external gelation, cross-linking agent was dissolved in the aqueous gelling bath outside. When added the mixture of the emulsions to Ca2+ solution, alginate–Ca beads were formed to encapsulate the emulsions and further impacted pH-responsive release of VD3 in stomach and rapid release of it in small intestine.
2. Materials and methods
2.1. Materials
Zein (Z0001) was purchased from Tokyo Chemistry Industry, Co., Ltd. (Tokyo, Japan). VD3 (99%) and tannin acid was purchased from Aladdin Chemistry Co., Ltd. Alginate and CaCl2·2H2O was obtained from the Sinopharm Chemical Reagents Co., Ltd. (Shanghai, China). Medium chain triglyceride (MCT) was purchased from Boxing Chemical Reagent Co. Ltd. (Wuhan, China). Other chemicals used were of analytical grade. All the solutions used in the experiments were prepared using ultrapure water through a Millipore (Millipore, Milford, MA, USA) Milli-Q water purification system.2.2. Preparation of zein–TA/Ca2+ NPs
All solutions were freshly prepared for immediate use. The standard preparation process was described as follows: zein (10 mg mL−1, 20 mg mL−1 and 30 mg mL−1) was dissolved in 75% alcohol-aqueous solution. Then 1 mL of the above mentioned solution was rapidly added into 9 mL of ultrapure water. Next, 100 μL of TA solution (24 mM) was added and the dispersion was briefly vortexed. The solution was heated in a water bath at 45 °C for 1 h with slow stirring. Following this, 100 μL of fresh metal solution (25 mM, 50 mM, 75 mM, 100 mM or 125 mM of CaCl2 solution) was added and the dispersion was vortexed. The product was then purified by successive dialysis (MWCO 3500) against deionized water for 36 h. The final product was freeze-dried and kept in a desiccator until use.2.3. Characterizations of zein–TA/Ca2+ NPs
2.4. Preparation of Pickering emulsions
The prepared zein–TA/Ca2+ NPs were utilized to prepared Pickering emulsions. The emulsions were formulated at oil/water ratio of 0.2. In brief, 20 mL of the zein–TA/Ca2+ NPs suspensions (with zein final concentration of 0.1%, 0.2% or 0.3%) in a glass vial, and 4 mL of MCT (with or without VD3) was added to the dispersion. Then the resultant mixtures were sheared using an IKA Ultra-Turrax T25 homongenizer at 6000 rpm for 5 min at room temperature. The obtained emulsions were directly subject to analysis or storage stability experiments.2.5. Morphology observation
The microstructures of the freshly formed emulsions were visualized by using static micro particle image analyzers (Winner 99D, China) with a 10 or 20× objective. The samples were diluted with deionized water.2.6. Particle size distribution of Pickering emulsions
Droplet size distribution of various freshly prepared emulsions was determined at room temperature, using a Malvern Mastersizer 2000 (Malvern Instruments Ltd., Malvern, Worcestershire, U.K.). Volume mean diameter (d4,3) was calculated from particle size profiles of the Pickering emulsions according to previous study.472.7. Preparation and characterizations of alginate–Ca beads
Ten milliliters of zein–TA/Ca2+ NPs stabilized Pickering emulsions were mixed with ten milliliters alginate solution with different concentrations (1%, 2%, 3%, 4% or 4.5%). Then the above mixed solution was extruded through a 0.55 mm needle and was dripped into Ca2+ gelling solution to form oil-loaded alginate–Ca beads. The gelling solution was gently stirred with a magnetic stirrer to prevent the beads sticking together.48 The beads that formed then hardened for 30 min in the gelling bath. A digital camera was used to capture the images of the wet beads. Also the microstructure of the freshly formed beads was observed by optical microscopy.2.8. Release profile of VD3 from alginate–Ca beads
The prepared alginate–Ca beads were used for in vitro kinetic release test in different conditions. For a kinetic release test at pH 7.4, a certain amount of sample was re-suspended in phosphate buffer containing 0.2% Tween 20 to provide sink condition. The experiment was carried out under the water bath at 37 °C with shaken speed of 100 rpm. At each predetermined time interval, 1 mL of the supernatant containing the released VD3 was collected and freeze-dried. The VD3 percentage released was calculated as a function of time (up to 8 h).The accumulated release profiles of the beads in the SGI with digestive enzymes were obtained using the method as previously reported.49 The samples were first incubated in 50 mL of simulated gastric fluid (SGF) with 0.1% pepsin (w/v) for 0.5 h and the supernatant containing the released VD3 was collected and freeze-dried. Digestion was stopped by raising the pH to 7.4 using NaOH, and the sample was then centrifuged to separate aggregates from supernatant. While the precipitate was re-dispersed using 50 mL of simulated intestinal fluid (SIF) with 1.0% pancreatin (w/v) at 37 °C and digested for 6 h under mild stirring. After digestion, the supernatant was collected and used for VD3 measurement. All measurements were performed in three replicates.
3. Results and discussion
3.1. Preparation of zein–TA/Ca2+ NPs
In this work, we fabricated a novel kind of Pickering emulsions stabilized by colloidal particles based on Ca2+–TA coated zein NPs. The excess Ca2+ on the surface of NPs was used to initiate alginate interaction to form a second layer. In the case of external gelation, the mixture of the emulsions was added to Ca2+ solution and alginate–Ca beads were formed to encapsulate the emulsions and further impacted pH-responsive deliver of VD3 in stomach and rapid release of it in small intestine.For the preparation of zein–TA/Ca2+ NPs, we first formed zein NPs and then added TA into zein NPs solution. After that we heated the formed NPs to enhance their interaction. During incubation, it can be predicted that hydrogen bonds of intramolecular may be broken which may provide complexation sites for TA to interact.25 In addition, TA contains numerous terminal hydroxyl groups, which endow it stronger surface binding affinity to proteins than other polyphenols.34,35 As shown in Scheme 1, Ca2+ was used to coordinate bonded with –OH units of TA. Also Ca2+ might interact with –COOH groups of zein to harden the NPs. Moreover, the Ca2+ on the surface of the NPs would combine with alginate, which would provide a second protection of the loadings in emulsions (Scheme 1).
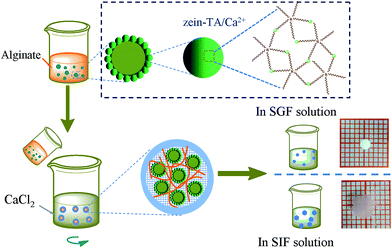 | ||
| Scheme 1 Illustration of the synthesis and structures of metal–TA coated zein NPs and the preparation for alginate–Ca gel. | ||
The influence of zein and Ca2+ concentrations on particle size, PDI and zeta potential in different formulations were summarized in Table 1. The particle size of zein–TA NPs without mental ion added was 75.4 ± 0.6, 105.2 ± 1.2, 129.4 ± 0.7 at zein concentration of 0.1%, 0.2%, 0.3% respectively (data was not shown). After connected by mental ions, the particle size varied with calcium concentration added. For each zein concentration, increase the amount of Ca2+ added led to a decrease trend of the particle size. Then further increase the Ca2+ concentration, the particle size increased until aggregation occurred. In addition, with the increase of zein concentration, the aggregation would occur at high Ca2+ concentration. The PDI in all formulations was less than 0.2, indicating that particles in these formulations may have uniform particle sizes. The addition of Ca2+ caused the decrease in zeta potential. As more Ca2+ was added and cross-linked with TA and zein, the zeta potential became less negative. In order to have more complexation sites for alginate to interact, we chose Z1–TA/Ca50, Z2–TA/Ca75 and Z3–TA/Ca100 for further study.
| Samples | Size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| a Z1–TA/Ca25, Z1–TA/Ca50 and Z1–TA/Ca75 represented zein–TA/Ca2+ NPs at zein concentration of 0.1% with the initial used metal ion concentration of 25 mM, 50 mM, and 75 mM respectively; Z2–TA/Ca25, Z2–TA/Ca50, Z2–TA/Ca75 and Z2–TA/Ca100 represented zein–TA/Ca2+ NPs at zein concentration of 0.2% with the initial used metal ion concentration of 25 mM, 50 mM, 75 mM and 100 mM respectively; Z3–TA/Ca25, Z3–TA/Ca50, Z3–TA/Ca75, Z3–TA/Ca100 and Z3–TA/Ca125 represented zein–TA/Ca2+ NPs at zein concentration of 0.3% with the initial used metal ion concentration of 25 mM, 50 mM, 75 mM, 100 mM and 125 mM respectively. The number labeled after Z or Ca means the different concentration of zein or Ca2+ used. | |||
| Z1–TA/Ca25 | 81.2 ± 1.6 | 0.15 ± 0.04 | −37.1 ± 0.9 |
| Z1–TA/Ca50 | 78.2 ± 2.3 | 0.19 ± 0.01 | −29.5 ± 1.3 |
| Z1–TA/Ca75 | Large aggregates | ||
| Z2–TA/Ca25 | 131.1 ± 1.1 | 0.11 ± 0.01 | −32.5 ± 0.8 |
| Z2–TA/Ca50 | 103.5 ± 0.9 | 0.18 ± 0.03 | −28.9 ± 1.0 |
| Z2–TA/Ca75 | 123.5 ± 0.9 | 0.14 ± 0.04 | −25.1 ± 0.6 |
| Z2–TA/Ca100 | Large aggregates | ||
| Z3–TA/Ca25 | 164.4 ± 1.0 | 0.12 ± 0.03 | −30.7 ± 0.8 |
| Z3–TA/Ca50 | 132.4 ± 2.1 | 0.14 ± 0.03 | −27.9 ± 0.6 |
| Z3–TA–Ca75 | 131.7 ± 1.1 | 0.10 ± 0.02 | −25.5 ± 0.5 |
| Z3–TA–Ca100 | 151.7 ± 4.0 | 0.13 ± 0.02 | −23.3 ± 0.7 |
| Z3–TA–Ca125 | Large aggregates | ||
3.2. Characterization of zein–TA/Ca2+ NPs
Fig. 1 showed the TEM images and typical size distribution profiles of zein–TA NPs and zein–TA/Ca2+ NPs. As shown in the TEM images (Fig. 1a–c), zein–TA NPs without Ca2+ shared features of a spherical shape, but most of the particles were clumped and connected to each other. After zein–TA NPs were connected by metal ions, it was possible to see individual NPs clearly with well-defined spherical shape and homogeneous distribution (Fig. 1d–i). In addition, the particle size increased with zein and Ca2+ concentration increased.Then, FT-IR method was used to verify the existence of the specific intermolecular interactions between Ca2+, TA and zein components in the metal/TA coated NPs. The representative spectra of zein, TA, zein–TA NPs and zein–TA/Ca2+ NPs were shown in Fig. 2. In the infrared spectra, a characterization peak was in the range of 3200–3400 cm−1, indicating the hydrogen bonding.49 The O–H stretching band of the hydroxyl groups in zein and TA was at 3420 cm−1 and 3398 cm−1, respectively, and shifted to 3347 cm−1 and 3317 cm−1 in zein–TA NPs and zein–TA/Ca2+ NPs respectively, suggesting the hydrogen bonding was formed between TA and zein. The amide I band of zein at 1648 cm−1 demonstrated a prominent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, while the amide II band at 1546 cm−1 demonstrated C–N stretching.50 Comparing the spectra of zein with zein–TA NPs or zein–TA/Ca2+ NPs, the band of amide I and amide II group shifted to 1658 cm−1 and 1533 in zein/TA NPs or zein–TA/Ca2+ NPs, indicating that the electrostatic interaction was an intermolecular force between zein and TA. Seen from the FTIR spectrum of all of zein–TA/Ca2+ NPs, the reduced intensity of the HO–C stretching peak at 1202 cm−1 also indicated that the phenolic groups coordinated with metal ions peak when compared with the spectrum of the zein–TA sample and TA.51
O stretching, while the amide II band at 1546 cm−1 demonstrated C–N stretching.50 Comparing the spectra of zein with zein–TA NPs or zein–TA/Ca2+ NPs, the band of amide I and amide II group shifted to 1658 cm−1 and 1533 in zein/TA NPs or zein–TA/Ca2+ NPs, indicating that the electrostatic interaction was an intermolecular force between zein and TA. Seen from the FTIR spectrum of all of zein–TA/Ca2+ NPs, the reduced intensity of the HO–C stretching peak at 1202 cm−1 also indicated that the phenolic groups coordinated with metal ions peak when compared with the spectrum of the zein–TA sample and TA.51
To further confirm the surface chemical information of the NPs, XPS analysis was performed. Fig. 3 displayed the survey scan spectrum of zein–TA/Ca2+ NPs, in which C 1s, O 1s and N 1s core-levels existed obviously. Three peak components of C 1s core-level photoelectron spectrum located at 284.2 eV, 285.5 eV, and 287.3 eV (Fig. 3c), which are coordinated to C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or O–C
O or O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. From Fig. 3b, Ca 2p signal was observed in the survey scan spectrum of zein–TA/Ca2+. In addition, the C/O ratio of zein, zein–TA/Ca2+ were obtained to be 4.18 and 3.23, respectively (Table 2). As is well-known, TA was rich in oxygen, about 43.27%, much higher than in zein which also identified the metal–phenolic films coating on zein NP.52
O group. From Fig. 3b, Ca 2p signal was observed in the survey scan spectrum of zein–TA/Ca2+. In addition, the C/O ratio of zein, zein–TA/Ca2+ were obtained to be 4.18 and 3.23, respectively (Table 2). As is well-known, TA was rich in oxygen, about 43.27%, much higher than in zein which also identified the metal–phenolic films coating on zein NP.52
| Samples | C | O | N | S | Ca |
|---|---|---|---|---|---|
| a Z1 represented zein NPs at zein concentration of 0.1%; Z1–TA/Ca50 represented zein–TA/Ca2+ NPs at zein concentration of 0.1% with the initial used metal ion concentration of 50 mM. The number labeled after Z or Ca means the different concentration of zein or Ca2+ used. | |||||
| Z1 | 68.65 | 16.44 | 14.34 | 0.57 | — |
| Z1–TA/Ca50 | 67.30 | 20.83 | 11.25 | 0.52 | 0.12 |
3.3. Characterization of zein–TA/Ca2+ NPs based Pickering emulsions
VD3 is a kind of liposoluble vitamin which is very sensitive to various environmental factors i.e., light, heat, and oxygen and could rapidly induce isomerization or oxidation and then decrease its physiological benefits.49,53 Additionally, the oxidation of lipids adversely affects the property of VD3. So in this study, we used TA, a kind of polyphenols, to surface coated zein NPs to impact the antioxidant activity of zein NPs. In addition, according to our precious work, TA in the NPs adsorbed at the oil/water interface was able to provide great protection again UV light-induced degradation of VD3.29In this study, Z1–TA/Ca50, Z2–TA/Ca75 and Z3–TA/Ca100 NPs were used to support the formation of stable Pickering emulsions. First, we evaluated the influence of zein and Ca2+ concentration in the aqueous phase on the microstructure and the droplet size distribution of the fresh emulsions. Table S1† showed the d4,3 value of droplets in different sets of fresh zein–TA/Ca2+ NPs stabilized emulsions, determined using water as the dispersing solvent. For the fresh prepared emulsions, we can see that increasing concentration of zein and Ca2+ resulted in a reduction in d4,3 first and then followed by an increase trend (Table S1†), with the droplet size widely ranging from 34.64 to 101.91 μm.
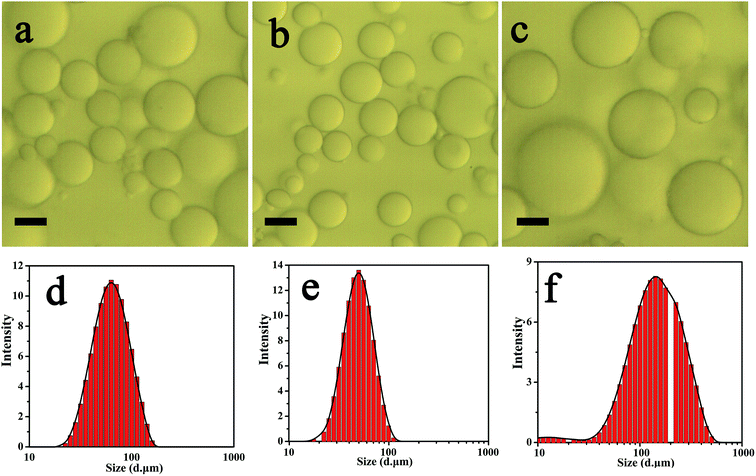
The flocculated state of oil droplets in the fresh Pickering emulsions were evaluated using microscope, as displayed in Fig. 4. As expected, the droplet size of these emulsions stabilized by zein–TA/Ca2+ NPs considerably varied with zein and Ca2+ concentration. For all the formulations, it can be observed that most of the droplets were present in the separated and unflocculated form (Fig. 4a–c).
Fig. 4d–f showed the typical droplet size distribution profile of the various fresh emulsions as a function of zein and Ca2+ concentration. All the emulsions fresh prepared at varying concentration exhibited a similarly monomodal size distribution profile. This indicated that all the emulsions were present in the unflocculated form which was corroborated by the microstructural observation of the emulsions using optical microscopy.
3.4. Preparation and characterizations of alginate–Ca beads
In order to impact a second protection and pH responsible release of VD3, we further used alginate–Ca gels to cover the emulsions. First, alginate was mixed with the fresh prepared emulsions, forming a second layer between Ca2+ on the surface of zein–TA/Ca2+ NPs. Then the mixture was slowly added into Ca2+ solution to form alginate–Ca beads.It was expected that the concentration of alginate solutions would affect the stability of the emulsions as well as the formation of the gel beads. Thus, this work was undertaken to determine the optimal concentrations of alginate for making the emulsions with the highest stability. In Fig. 5c–g, it can be observed that most of the droplets were present in the separated and unflocculated form, and the droplet size progressively decreased with increasing the alginate concentration. Moreover, the increase in the concentration of alginate from 1% to 4.5% led to an increase in stability of the emulsions (Fig. 5a–b). After storage for 3 days, the emulsions became unstable, containing water phase in the bottom except the emulsions with 4.5% alginate (Fig. 5b).
Then we used emulsions with 4.5% alginate to prepare alginate–Ca beads. The surface morphology of microparticles was observed by optical light microscopy. In Fig. 6a–e, we could see the droplets were homogenously entrapped within the gel network. With the increase of alginate concentration, the amount of oil droplet on the surface decreased and almost all particles were encapsulated by the gel.
 | ||
| Fig. 6 (a)–(e) Microscopy images of Pickering emulsions stabilized Z1–TA/Ca50 NPs encapsulated by alginate with the concentration of 4.5% in alginate–Ca gel. The scale bar is 20 μm. | ||
3.5. Release of VD3 from alginate–Ca beads
The intestinal-targeted characteristics of alginate–Ca beads are investigated by studying the dissolution of beads in SGF and SIF. Fig. 7 showed the release rate of alginate–Ca beads after immersed in pH 1.2 SGF and pH 7.4 SIF. As can be seen from the picture, the alginate–Ca beads did not show significant changes in shape in SGF, while exist dramatic increase in beads size in SIF. If prolonged the incubation time, the beads trended to dissolve. | ||
| Fig. 7 Photographs of alginate–Ca beads encapsulating Pickering emulsions stabilized by Z1–TA/Ca50 NPs (a) or Z2–TA/Ca75 NPs (b) in gastric fluid (up) or intestinal fluid (down). | ||
Then we monitored the kinetic release profile of beads in PBS and the cumulative release profile in SGI with digestive enzymes. In PBS medium, all formulations showed a first-order release profile, with biphasic kinetic releasing trend, that is, a burst effect within 2 h followed by a sustained release for up to 6 h with nearly 90% and 80% VD3 released for emulsions stabilized by Z1–TA/Ca50 and Z2–TA/Ca75. Under the SGI condition with digestive enzymes, after incubation at 37 °C in SGF for 30 min, only about 18% of the VD3 was released from emulsions stabilized by Z1–TA/Ca50 or Z2–TA/Ca75. When transferred to the SIF (pH 7.4), at most 95% of VD3 was released from the polymeric matrix within 6 h for emulsions stabilized by Z1–TA/Ca50. In contrast, 85% of the VD3 was detected in the releasing medium for emulsions stabilized by Z2–TA/Ca75. For emulsions stabilized Z2–TA/Ca75 NPs, more Ca2+ could interact with more alginate forming robuster coating which provide a relative slow release rate.
It has been reported that alginate gel is pH sensitive, which shrinks at lower pH and swells at higher pH. As known, VD3 will decrease the stability in acidic conditions. This property of alginate beads was contributed to prominent protection on VD3 against digestion in the gastric fluid, together with an increased amount of VD3 being delivered to the small intestine (Fig. 8).
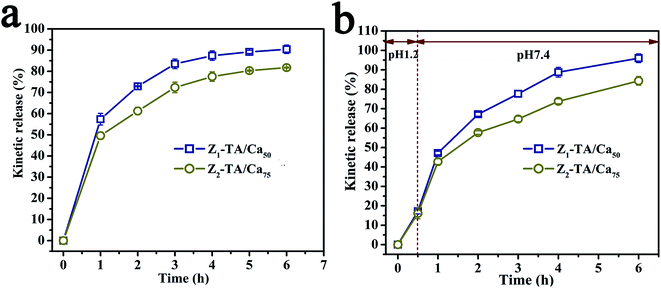 | ||
| Fig. 8 Kinetic release of VD3 from alginate–Ca beads in pH 7.4 media (a). Release of VD3 in SGF and SIF from alginate–Ca beads (b). | ||
4. Conclusion
In this work, we fabricated a novel kind of Pickering emulsions stabilized by zein–TA/Ca2+ NPs. This kind of particles was irreversibly anchored at the oil–water interface to form particle-based network architecture therein, producing ultrastable O/W Pickering emulsions. The excess Ca2+ on the surface of NPs was used to initiate alginate interaction to form a second layer. In the case of external gelation, cross-linking agent (Ca2+) was dissolved in the aqueous gelling bath outside. When added the mixture of the emulsions to Ca2+ solution, alginate–Ca beads were formed. Compared with previous VD3 delivery system about NPs49,54 or nanoemulsions,55 this alginate beads demonstrated the pH-responsive deliver of VD3 in stomach and rapid release of it in small intestine to provide a comprehensive protection.Acknowledgements
This work was financially supported by the Fundamental Research Funds for the Central Universities (Program No. 2662016PY092). The authors would like to express their sincere gratitude to many conveniences offered by colleagues of Key Laboratory of Environment Correlative Dietology of Huazhong Agricultural University.References
- M. V. Lomova, G. B. Sukhorukov and M. N. Antipina, ACS Appl. Mater. Interfaces, 2010, 2, 3669–3676 CAS
.
- J. Tang, P. J. Quinlan and K. C. Tam, Soft Matter, 2015, 11, 3512–3529 RSC
.
- E. Dickinson, Trends Food Sci. Technol., 2012, 24, 4–12 CrossRef CAS
.
- Z. M. Gao, X. Q. Yang, N. N. Wu, L. J. Wang, J. M. Wang, J. Guo and S.-W. Yin, J. Agric. Food Chem., 2014, 62, 2672–2678 CrossRef CAS PubMed
.
- M. Bertoldo, M. B. Coltelli, T. Messina, S. Bronco and V. Castelvetro, ACS Biomater. Sci. Eng., 2016, 2, 677–686 CrossRef CAS
.
- A. V. Sadovoy, M. V. Lomova, M. N. Antipina, N. A. Braun, G. B. Sukhorukov and M. V. Kiryukhin, ACS Appl. Mater. Interface., 2013, 5, 8948–8954 CrossRef CAS PubMed
.
- X. Y. Wang and M. C. Heuzey, Langmuir, 2016, 32, 929–936 CrossRef CAS PubMed
.
- E. L. Sharp, H. Al-Shehri, T. S. Horozov, S. D. Stoyanov and V. N. Paunov, RSC Adv., 2013, 4, 2205–2213 RSC
.
- R. von Klitzing, D. Stehl, T. Pogrzeba, R. Schomäcker, R. Minullina, A. Panchal, S. Konnova, R. Fakhrullin, J. Koetz and H. Möhwald, Adv. Mater. Interfaces, 2016 DOI:10.1002/admi.201600435
.
- K. Larson-Smith and D. C. Pozzo, Langmuir, 2012, 28, 11725–11732 CrossRef CAS PubMed
.
- B. V. Parakhonskiy, A. M. Yashchenok, M. Konrad and A. G. Skirtach, Adv. Colloid Interface Sci., 2014, 207, 253–264 CrossRef CAS PubMed
.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 2539–2547 CrossRef CAS
.
- J. S. Weston, R. E. Jentoft, B. P. Grady, D. E. Resasco and J. H. Harwell, Ind. Eng. Chem. Res., 2015, 54, 4274–4284 CrossRef CAS
.
- M. Pan, L. Rosenfeld, M. Kim, M. Xu, E. Lin, R. Derda and S. K. Y. Tang, ACS Appl. Mater. Interfaces, 2014, 6, 21446–21453 CAS
.
- M. Williams, B. Olland, S. P. Armes, P. Verstraete and J. Smets, J. Colloid Interface Sci., 2015, 460, 71–80 CrossRef CAS PubMed
.
- M. Nawaz, W. Miran, J. Jang and D. S. Lee, Appl. Catal. B, 2017, 203, 85–95 CrossRef CAS
.
- X. He, X. Ge, H. Liu, H. Zhou and Z. Zhang, Colloids Surf., A, 2007, 301, 80–84 CrossRef CAS
.
- J. E. Norton and I. T. Norton, Soft Matter, 2010, 6, 3735–3742 RSC
.
- F. Liu and C. H. Tang, J. Agric. Food Chem., 2013, 61, 8888–8898 CrossRef CAS PubMed
.
- Z. Wei, C. Wang, S. Zou, H. Liu and Z. Tong, Polymer, 2012, 53, 1229–1235 CrossRef CAS
.
- Y. Tan, K. Xu, C. Liu, Y. Li, C. Lu and P. Wang, Carbohydr. Polym., 2012, 88, 1358–1363 CrossRef CAS
.
- L. J. Wang, Y. Q. Hu, S. W. Yin, X. Q. Yang, F. R. Lai and S. Q. Wang, J. Agric. Food Chem., 2015, 63, 2514–2524 CrossRef CAS PubMed
.
- Y. Tan, K. Xu, C. Niu, C. Liu, Y. Li, P. Wang and B. P. Binks, Food Hydrocolloids, 2014, 36, 70–75 CrossRef CAS
.
- H. Liang, Q. Huang, B. Zhou, L. He, L. Lin, Y. An, Y. Li, S. Liu, Y. Chen and B. Li, J. Mater. Chem. B, 2015, 3, 3242–3253 RSC
.
- Y. Zou, J. Guo, S. W. Yin, J. M. Wang and X. Q. Yang, J. Agric. Food Chem., 2015, 63, 7405–7414 CrossRef CAS PubMed
.
- H. Liang, B. Zhou, L. He, Y. An, L. Lin, Y. Li, S. Liu, Y. Chen and B. Li, RSC Adv., 2015, 5, 13891–13900 RSC
.
- J. W. de Folter, M. W. van Ruijven and K. P. Velikov, Soft Matter, 2012, 8, 6807–6815 RSC
.
- A. R. Patel, J. S. ten-Hoorn, J. Hazekamp, T. B. J. Blijdenstein and K. P. Velikov, Soft Matter, 2013, 9, 1428–1436 RSC
.
- H. Liang, Y. Pei, J. Li, W. Xiong, Y. He, S. Liu, Y. Li and B. Li, RSC Adv., 2016, 6, 31374–31385 RSC
.
- C. Li, L. Xing and S. Che, Dalton Trans., 2012, 41, 3714–3719 RSC
.
- H. Liang, J. Li, Y. He, W. Xu, S. Liu, Y. Li, Y. Chen and B. Li, ACS Biomater. Sci. Eng., 2016, 2, 317–325 CrossRef CAS
.
- Y. N. Zhao, J. Gu, S. Jia, Y. Guan and Y. Zhang, Soft Matter, 2016, 12, 1085–1092 RSC
.
- M. Shin, K. Kim, W. Shim, J. W. Yang and H. Lee, ACS Biomater. Sci. Eng., 2016, 2, 687–696 CrossRef CAS
.
- T. Shutava, M. Prouty, D. Kommireddy and Y. Lvov, Macromolecules, 2005, 38, 2850–2858 CrossRef CAS
.
- M. Andjelković, J. Van Camp, B. De Meulenaer, G. Depaemelaere, C. Socaciu, M. Verloo and R. Verhe, Food Chem., 2006, 98, 23–31 CrossRef
.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244–4258 RSC
.
- H. Liang, B. Zhou, J. Li, W. Xu, S. Liu, Y. Li, Y. Chen and B. Li, Colloids Surf., B, 2015, 136, 1224–1233 CrossRef CAS PubMed
.
- Y. Fang, S. Al-Assaf, G. O. Phillips, K. Nishinari, T. Funami and P. A. Williams, Carbohydr. Polym., 2008, 72, 334–341 CrossRef CAS
.
- I. Matai and P. Gopinath, ACS Biomater. Sci. Eng., 2015, 2, 213–223 CrossRef
.
- K. I. Draget, G. Skjåk-Bræk and O. Smidsrød, Int. J. Biol. Macromol., 1997, 21, 47–55 CrossRef CAS PubMed
.
- Y. Liu, S. Chen, L. Zhong and G. Wu, Radiat. Phys. Chem., 2009, 78, 251–255 CrossRef CAS
.
- J. A. Luckanagul, K. Metavarayuth, S. Feng, P. Maneesaay, A. Y. Clark, X. Yang, A. J. García and Q. Wang, ACS Biomater. Sci. Eng., 2016, 2, 606–615 CrossRef CAS
.
- L. Mei, F. He, R. Q. Zhou, C. D. Wu, R. Liang, R. Xie, X. J. Ju, W. Wang and L. Y. Chu, ACS Appl. Mater. Interfaces, 2014, 6, 5962–5970 CAS
.
- K. Y. Lee and T. R. Heo, Appl. Environ. Microbiol., 2000, 66, 869–873 CrossRef CAS PubMed
.
- Y. Zhang, X. Jia, L. Wang, J. Liu and G. Ma, Ind. Eng. Chem. Res., 2011, 50, 4106–4112 CrossRef CAS
.
- M. Marquis, V. Alix, I. Capron, S. Cuenot and A. Zykwinska, ACS Biomater. Sci. Eng., 2016, 2, 535–543 CrossRef CAS
.
- L. J. Wang, Y. Q. Hu, S. W. Yin, X. Q. Yang, F. R. Lai and S. Q. Wang, J. Agric. Food Chem., 2015, 63, 2514–2524 CrossRef CAS PubMed
.
- M. Marquis, V. Alix, I. Capron, S. Cuenot and A. Zykwinska, ACS Biomater. Sci. Eng., 2016, 2, 535–543 CrossRef CAS
.
- Y. Luo, Z. Teng and Q. Wang, J. Agric. Food Chem., 2012, 60, 836–843 CrossRef CAS PubMed
.
- B. Zhou, Y. Li, H. Deng, Y. Hu and B. Li, Colloids Surf., B, 2014, 116, 432–438 CrossRef CAS PubMed
.
- J. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, Y. Yan, K. Peter, D. von Elverfeldt, C. E. Hagemeyer and F. Caruso, Angew. Chem., Int. Ed. Engl., 2014, 53, 5546–5551 CrossRef CAS PubMed
.
- B. Zhou, X. Jin, H. Liang, J. Li, S. Liu, Y. Li, Y. Chen and B. Li, RSC Adv., 2015, 5, 26965–26971 CAS
.
- J. M. Ballard, L. Zhu, E. D. Nelson and R. A. Seburg, J. Pharm. Biomed. Anal., 2007, 43, 142–150 CrossRef CAS PubMed
.
- Z. Teng, Y. Luo and Q. Wang, Food Chem., 2013, 141, 524–532 CrossRef CAS PubMed
.
- M. Guttoff, A. H. Saberi and D. J. McClements, Food Chem., 2015, 171, 117–122 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21755a |
| This journal is © The Royal Society of Chemistry 2016 |