Selective hydrogenation of furfural on Ru/Al-MIL-53: a comparative study on the effect of aromatic and aliphatic organic linkers†
Jie Yang*a,
Jianjun Maa,
Qingqing Yuanb,
Pei Zhangb and
Yejun Guan*b
aSchool of Mathematics and Physics, Shanghai University of Electric Power, Shanghai, 200090, China. E-mail: yj_7667@aliyun.com
bShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062, China
First published on 21st September 2016
Abstract
Two Al-MIL-53 materials containing aromatic and aliphatic linkers were prepared by using 1,4-benzenedicarboxylic acid (BDC) and adipic acid (ADP) as organic precursors, respectively. The resulting materials were characterized by BET, XRD, IR and TG. The characterization results indicated that the framework containing an aromatic ring (Al-MIL-53-BDC) shows a much higher surface area (761 m2 g−1) than the analogue with an aliphatic linker (Al-MIL-53-ADP, 4 m2 g−1). Al-MIL-53-BDC showed a larger adsorption capacity towards organic substrates such as furfural due to its higher surface area. Both materials can support Ru nanoparticles efficiently via a simple deposition–reduction method. XPS and TEM results suggested that Ru particles are predominantly oxidized with a particle size in the range of 1–4 nm. TPR results indicated that the oxidized Ru nanoparticles on Al-MIL-53-BDC can be reduced at around room temperature, forming metallic Ru nanoparticles which serve as the active sites for the catalytic hydrogenation of furfural under mild conditions. Full conversion of furfural and 100% selectivity to furfuryl alcohol can be achieved at 20 °C and 0.5 MPa H2 on Ru/Al-MIL-53-BDC.
1. Introduction
The sharp increase in the demand for fuel and chemicals requires the development of economical and sustainable production of fuels and chemicals, which has motivated extensive research on biomass conversion, especially for the use of lignocelluloses.1 Furfural (FUR) is a heteraromatic furan ring with an aldehyde functional group, which is produced by the acid-catalyzed digestion of hemicellulose-rich agricultural wastes.2 Therefore, FUR has been identified as one of the top-10 chemical platforms directly derived from biomass.3 Of the world production of FUR 60–70% is converted to furfuryl alcohol (FA) by selective hydrogenation because the latter compound has found broad applications in fine chemicals, polymer industry, and foundry technology.2 FA can be prepared from FUR by vapor-phase reaction or by liquid-phase reaction, with the former one being widely employed in industry by using reduced Cu catalyst.4,5 The hydrogenation of FUR to FA is still challenging because many side-products or coke are formed and the Cu catalyst deactivates rapidly during the reaction.6,7 Therefore, alternative catalytic approaches like liquid phase hydrogenation using metals other than Cu are also being explored.8–14 In principle, the selectivity is strongly related to the support, reaction solvent and temperature.9Metal–organic frameworks (MOFs) consisting of metal centers or inorganic clusters bridged by organic linkers through metal–ligand coordination bonds, have found wide applications either as catalysts by themselves or as support.15,16 Compared with conventional porous inorganic materials, MOFs show unique property as support of metal nanoparticles due to the strong host–guest interaction between the framework and metal nanoparticles,16 leading to enhanced catalytic activities of hosted metal nanoparticles.17–21 We have recently loaded Ru nanoparticles onto several Zr-MOFs with different aromatic linkers such as 1,4-benzenedicarboxylic acid (BDC), 2,6-naphthalenedicarboxylic acid (NDC), and 4,4′-biphenyldicarboxylic acid (BPDC).14 These Ru nanoparticles show excellent activity in FUR hydrogenation and good selectivity to FA. The activity can be linked to the presence of metallic Ru nanoparticles, the ratio of which is associated to the nature of aromatic linkers. In this study, we further extend this study by comparing the effect of aromatic linker and aliphatic counterparts on the hydrogenation activity of Ru nanoparticles. To this end, two Al-MIL-53 materials (Fig. 1), namely, Al-MIL-53-BDC and Al-MIL-53-ADP (Al-adipate, Al(OH)(O2C–C4H8–CO2)) were synthesized and used as supports of Ru nanoparticles. The results suggest that two materials show distinct textural properties which lead to various adsorption properties of FUR and catalytic activities in FUR hydrogenation. The Al-MIL-53-BDC with highly porous framework and large surface area benefits the hydrogenation reaction due to enhanced FUR adsorption and improved Ru dispersion.
2. Experimental
2.1 Synthesis of metal–organic frameworks
The Al-MIL-53-BDC (ADP) were prepared under hydrothermal conditions according to literature.18,222.2 Preparation of Ru catalyst
The supported Ru catalysts were prepared by deposition–reduction of Ru on the Al-MIL-53s. Typically, 2.24 mL of RuCl3 solution (Ru: 13.4 mg mL−1) was diluted into 60 mL of deionized water. Then, 0.97 g of vacuum-dried support was added to the RuCl3 solution and thoroughly stirred for 4 h. In order to reduce the Ru precursor, 100 μL of N2H4·H2O was added. The solid was filtered and dried at 70 °C under vacuum overnight. The catalysts were pretreated in flowing H2 at 200 °C for 2 h and cooled to room temperature in flowing nitrogen. The catalyst was stored in air under ambient conditions.2.3 Characterization
Powder X-ray diffraction (PXRD) patterns of all samples were recorded on a Rigaku-Ultima diffractometer using a Cu Kα radiation source (λ = 0.15432 nm) operated at 35 kV and 25 mA in the 2θ range from 5° to 80°. Fourier transform infrared (FT-IR) spectra were collected on a Nicolet Fourier transform infrared spectrometer (NEXUS 670). Thermal gravimetric analysis (TGA) curves were obtained on a PerkinElmer Pyris Diamond Thermogravimetric Differential Thermal/Analyzer. Samples were heated from room temperature to 800 °C with a heating rate of 10 °C min−1 under an air flow. The pore textural properties, including BET surface area and pore volume, were recorded on a Micromeritics Tristar 3000 adsorption analyzer at −196 °C. Prior to the adsorption measurements, the samples were degassed in situ under vacuum at 150 °C for 10 h. BET surface areas were calculated in the adapted pressure range of P/P0 = 0.01–0.1. Transmission electron microscopy (TEM) images were taken on a FEI Tecnai G2 F30 microscope operated at 300 kV. The Ru loading was determined by a Thermo Elemental IRIS Intrepid II XSP inductively coupled plasma emission spectrometer (ICP-AES). Prior to analysis, an amount of catalyst was dissolved in 10 mL of royal water by heating in an autoclave at 180 °C for 6 h. XP spectra were recorded on a Kratos AXIS Ultra DLD spectrometer with a monochromated Al Kα radiation source. The survey spectra were recorded with a pass energy of 160 eV; high-resolution spectra with a pass energy of 40 eV to identify the chemical state of each element. All the binding energies (BEs) were referred to the C 1s neutral carbon peak at 284.6 eV. H2-TPR experiments were performed on a XianQuan (Tianjing) TP-5078 TPR/TPD analyzer equipped with a thermal conductivity detector (TCD). 100 mg of sample was placed in a quartz tubular reactor and pretreated in a He stream, heated to 200 °C at a rate of 10 °C min−1, and held at this temperature for 60 min. After cooling to room temperature, a gaseous mixture of 5% H2 in Ar was fed at a flow rate of 30 mL min−1 and the sample temperature was increased to 150 °C with ramp rate of 10 °C min−1.2.4 Catalytic activity tests
A Teflon-lined (120 mL) steel batch reactor was used to carry out the liquid phase hydrogenation reactions. Desired amount of as-prepared catalyst was loaded into the reactor with 9.9 mL of H2O and 100 μL of FUR. The reactor was purged five times with H2 and then pressurized with 5 bar H2. The reaction vessel was kept at room temperature (20 °C) and stirred for different periods. The products were diluted with ethanol and analyzed on a Tianmei 7900 GC equipped with a DM-FFAP capillary column (30 m length, 0.25 μm film thickness and 0.25 mm internal diameter). In all cases, FA was observed as the only product. By variation of the stirring speed, it was verified that there were no diffusional limitations during the catalytic tests.3. Results and discussion
3.1 Characterization of Al-MIL-53s
Two Al-based MOFs were prepared, namely MIL-53-BDC and MIL-53-ADP, using different organic linkers including BDC and ADP, respectively. Fig. 2 displays the XRD patterns of the activated materials. The diffraction pattern of Al-MIL-53-BDC suggests the coexistence of two structures with both large-pore (lp, 8.5 × 8.5 Å2) and narrow-pore (np, 2.6 × 13.6 Å2).23 Al-MIL-53-ADP shows somewhat similar diffraction pattern to an early report by Reinsch and coworkers,22 who have determined its structure by combination of forcefield-based computations and Rietveld refinement of the PXRD data. They found that Al-MIL-53-ADP has a dense structure and the packing of channels (with diameter ca. 3.2 Å) is substantially different compared to that of MIL-53-BDC. Some unassigned weak diffraction peaks are present in the XRD pattern, suggesting the presence of trace impurities in the Al-MIL-53-ADP. At this stage, we are not able to identify the structure of these impurities.Fig. 3 shows the IR spectra of two Al-MIL-53s. Peaks from 1400 to 1650 cm−1 are attributed to the carboxylic function.23 Several peaks between 3000 to 2800 cm−1 can be assigned to the C–H stretching vibrations of the aliphatic linker –CH2–.22 Broad peaks above 3500 cm−1 are due to the Al–OH groups perturbed by adsorbed water molecules. It should be noted that neither solvent nor free carboxylic acid precursors are present in the activated samples according to the IR spectra.
The surface areas of both materials were determined by liquid nitrogen adsorption (Fig. 4). The surface areas of Al-MIL-53-BDC and Al-MIL-53-ADP are 761 and 4 m2 g−1, respectively. The surface area of Al-MIL-53 is close to our previous study.18 The very low surface area of Al-MIL-53-ADP is not surprising taking into account of its very small channel diameter ca. 3.2 Å as determined by pore size distribution analysis carried out on the structural model.22 Since the kinetic molecular size of N2 is ca. 3.6 Å,24 one can clearly see that the N2 molecules cannot enter into these channels. The thermal stability of Al-MIL-53-BDC and ADP upon calcination in air was investigated by TGA and the results are shown in Fig. 5. Both TG curves show two weight-loss steps. The first loss of 5.3% at temperature below 100 °C for Al-MIL-53-BDC is due to the desorption of adsorbed water, which is consistent with our previous finding.18 The corresponding loss of water for Al-MIL-53-ADP is 8.5%, which is close to the theoretical value.22 The second weight loss in TG curves points to the thermal stability of two materials. The decomposition temperatures of Al-MIL-53-BDC and ADP are 480 and 340 °C, respectively, suggesting that aromatic linker is superior to aliphatic counterparts for the formation of thermostable framework. This stabilization effect is probably related to the strong interaction between metal nodes and the carboxylate coordinated to π-conjugation.
The adsorption/desorption behavior of FUR on Al-MIL-53-BDC and ADP materials has also been studied by thermogravimetric analysis study. Before each measurement, the samples were dried and then transferred into a sealed vessel with liquid FUR allowing for the saturation of surface by FUR. It should be noted that we could not completely avoid the co-adsorption of water vapor in this experiment. The TGA curves of Al-MIL-53-BDC and ADP with FUR adsorbed are shown in Fig. 6. The first weight loss occurred in the range of 30 to 200 °C is ca. 35% for Al-MIL-53-BDC, suggesting that large amount of FUR can be adsorbed on the surface. In contrast, 12% of weight loss is observed for Al-MIL-53-ADP and the desorption is completed at ca. 100 °C. This result shows that FUR adsorbs more strongly on Al-MIL-53-BDC than on Al-MIL-53-ADP, likely due to the large surface are and pore size effect of the former one. Similar phenomenon has been found for phenol adsorption on Cr-MIL-53 and Cr-MIL-101 with different porous structures.24
3.2 Characterization of Ru/Al-MIL-53s
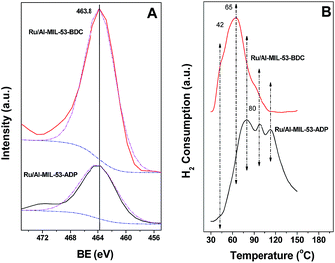
The liquid nitrogen adsorption isotherms of supported Ru catalysts are also shown in Fig. 4. The BET surface areas of Ru/Al-MIL-53-BDC and ADP calculated from the physisorption isotherms are 497 and 18 m2 g−1, respectively. The decrease in surface area of Al-MIL-53-BDC after loading Ru is expected according to previous study.14,18 For Ru/Al-MIL-53-ADP, the increased surface area might be associated to the intra crystalline pores resulted by the collapse of framework during the preparation as revealed by the XRD pattern of Ru loaded sample (Fig. S1†). The framework collapse might take place since the ruthenium salt is strongly acidic, leading to the cleavage of Al–OO–C bond in aliphatic MOFs. The Ru loading of Ru/Al-MIL-53-BDC and ADP is found to be 2.9 and 3.0 wt%, respectively. Fig. 7 shows typical HRTEM images of Ru/Al-MIL-53s. Homogeneously dispersed Ru nanoparticles are clearly observed on Ru/Al-MIL-53-BDC, with particle size ca. 3 nm. Similar particle size distribution is found for Ru/Al-MIL-53-ADP. However, these Ru nanoparticles are aggregated to each other on the external surface, therefore showing minute host–guest effect between the metal and framework. Our previous studies have shown that several parameters of MOFs including surface hydrophilicity, pore size, surface area and etc., play a sophisticated role in determining the size and electronic properties of supported metal nanoparticles.14,18Fig. 8A shows the XP spectra of Ru/MIL-53 with two linkers. The BE peak centered at 463.8 eV is assigned to the oxidized RuO2, suggesting the high oxidation tendency of metallic Ru upon exposure in air. Fig. 8B shows the H2-temperature programmed reduction (H2-TPR) of Ru/Al-MIL-53s in the temperature range of 30 to 150 °C. For both catalysts, a broad H2 consumption peak with different Tmax was observed. The reduction of these oxidized Ru species started at ca. 30 °C on Ru/Al-MIL-53-BDC, whereas it is 45 °C for Ru/Al-MIL-53-ADP. The Tmax of Ru/Al-MIL-53-BDC and ADP is 65 and 98 °C, respectively. These results suggest that Ru particles on Ru/Al-MIL-53-BDC can be reduced at much lower temperatures, which is critical for low temperature hydrogenation reactions. Our results have shown that the metallic Ru species is favorable for the hydrogenation of FUR in water.
3.3 Catalytic activity tests
FUR hydrogenation was carried out in water at room temperature at a H2 pressure of 0.5 MPa as previously reported.14 The Al-MIL-53 analogues both did not show any hydrogenation activity within 2 h under the reaction conditions investigated when 100 mg catalyst each was used (Table 1). The FUR conversion and turnover frequency (TOF) based on the moles of furfural converted per mole of Ru per hour are given in Table 1. In all cases, FA was observed as the only product. Full conversion of FUR was observed in 2 h when 100 mg of Ru/Al-MIL-53-BDC catalyst was used. This reactivity is much higher than that observed for Ru/Zr-MOFs, which showed less than 95% conversion (Ru/Zr-UiO-66) of FUR after reaction for 4 h under otherwise the same conditions.14 We then decreased the catalyst amount and shortened the reaction period to 1 h to estimate the TOF of Ru nanoparticles. The results showed in Table 1 suggested that a TOF ca. 20 h−1 was noticed, which is about twice of Ru/Zr-UiO-66. For Ru/MIL-53-ADP, only 44% FAL conversion was achieved after 2 h when 100 mg catalyst was used. Nevertheless, this reactivity is still higher than Ru supported on Zr-MIL-140s. These results suggest that the cheap Al based MOFs are more promising and economical than Zr-based materials as support of Ru nanoparticles for hydrogenation reactions.| Catalyst | Weight (mg) | Time (h) | XFURb (%) | SFAc (%) | TOFd (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: 100 μL of furfural in 9.9 mL of H2O, 0.5 MPa H2, 20 °C.b Conversion of furfural.c Selectivity to furfuryl alcohol (FA).d The TOF is defined as molFUR (molRu h)−1. | |||||
| Al-MIL-53-BDC | 100 | 2 | 0 | — | — |
| Al-MIL-53-ADP | 100 | 2 | 0 | — | — |
| 2.9Ru/Al-MIL-53-BDC | 25 | 1 | 12 | >99.9 | 20 |
| 50 | 1 | 21 | >99.9 | 18 | |
| 100 | 2 | 100 | >99.9 | — | |
| 3.0Ru/Al-MIL-53-ADP | 25 | 1 | 3.0 | >99.9 | 4.9 |
| 50 | 1 | 5.0 | >99.9 | 4.1 | |
| 100 | 2 | 44 | >99.9 | 8.9 | |
According to the TEM and XPS results, no significant differences in particle size and oxidation state of Ru nanoparticles could be distinguished when supported on Al-MIL-53-BDC and ADP. For both catalysts, Ru nanoparticles are in size of 2–4 nm and mainly in oxidized state when exposed in air. It should be noted that these oxidized Ru species on Al-MIL-53-BDC can be reduced at or close to room temperature as clearly shown by H2-TPR study. In contrast, the reduction of oxidized Ru species need higher temperature on Al-MIL-53-ADP. This result unambiguously points to the distinct ability of Ru species towards reduction in H2, which will affect the activity of Ru species in hydrogenation. On the other hand, the adsorption capacity of FUR on Al-MIL-53-BDC is almost trice of that on Al-MIL-53-ADP, which might be also beneficial for the hydrogenation process.25
4. Conclusions
The effects of aromatic and aliphatic linkers of MOFs in supporting Ru nanoparticles and their catalytic activities in selective hydrogenation were compared. To this end, two Al-MIL-53 materials with benzene-dicarboxylic acid and adipic acid as framework linkers were synthesized and used as the carrier of Ru nanoparticles. The loaded Ru nanoparticles in range of 1–4 nm are in predominantly oxidized phase when exposed to air. In situ reduction of oxidized Ru species might occur on Ru/Al-MIL-53-BDC according to H2-TPR study, therefore leading to very high activity in liquid phase hydrogenation of furfural. This study indicates that aromatic linkers are likely preferred as supports because of their high surface area which contributes to the metal dispersion and enhanced substrate adsorption.Acknowledgements
This work is financially supported by the Science and Technology Commission of Shanghai Municipality (13ZR1417900) and the National Natural Science Foundation of China (21203065).Notes and references
- B. Kamm, P. R. Gruber and M. Kamm, Biorefineries-Industrial Processes and Products: Status Quo and Future Directions, Wiley-VCH Verlag GmbH & Co., Weinheim, 2010 Search PubMed.
- H. E. Hoydonckx, W. M. Van Rhijn, W. Van Rhijn, D. E. De Vos and P. A. Jacobs, Furfural and Derivatives, Ullmann's Encyclo. Ind. Chem., 2012, vol. 16, pp. 285–313 Search PubMed.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sadaba and M. Lopez Granados, Energy Environ. Sci., 2016, 9, 1144–1189 CAS.
- G. Seo and H. Chon, J. Catal., 1981, 67, 424–429 CrossRef CAS.
- C. P. Jimenezgomez, J. A. Cecilia, D. Duranmartin, R. Morenotost, J. M. Meridarobles and P. Mairelestorres, J. Catal., 2016, 336, 107–115 CrossRef CAS.
- D. Liu, D. Zemlyanov, T. Wu, R. J. Lobo-Lapidusa, J. A. Dumesicc, J. T. Miller and C. L. Marshall, J. Catal., 2013, 299, 336–345 CrossRef CAS.
- H. Zhang, Y. Lei, A. J. Kropf, G. Zhang, J. W. Elam, J. T. Miller, F. Sollberger, F. Ribeiro, M. C. Akatay, E. A. Stach, J. A. Dumesic and C. L. Marshall, J. Catal., 2014, 317, 284–292 CrossRef CAS.
- H. X. Li, H. S. Luo, L. Zhuang, W. L. Dai and M. H. Qiao, J. Mol. Catal. A: Chem., 2003, 203, 267–275 CrossRef CAS.
- M. J. Taylor, L. J. Durndell, M. A. Isaacs, C. M. A. Parlett, K. Wilson, A. F. Lee and G. Kyriakou, Appl. Catal., B, 2016, 180, 580–585 CrossRef CAS.
- R. V. Maligal-Ganesh, C. Xiao, T. W. Goh, L.-L. Wang, J. Gustafson, Y. Pei, Z. Qi, D. D. Johnson, S. Zhang, F. Tao and W. Huang, ACS Catal., 2016, 6, 1754–1763 CrossRef CAS.
- X. Chen, L. Zhang, B. Zhang, X. Guo and X. Mu, Sci. Rep., 2016, 6, 28558 CrossRef CAS PubMed.
- M. Audemar, C. Ciotonea, K. de Olieveria Vigier, S. Royer, A. Ungureanu, B. Dragoi, E. Dumitriu and F. Jerome, ChemSusChem, 2015, 8, 1885–1891 CrossRef CAS PubMed.
- J. Chen, F. Lu, J. Zhang, W. Yu, F. Wang, J. Gao and J. Xu, ChemCatChem, 2013, 5, 2822–2826 CrossRef CAS.
- Q. Yuan, D. Zhang, L. Van Haandel, F. Ye, T. Xue, E. J. M. Hensen and Y. Guan, J. Mol. Catal. A: Chem., 2015, 406, 58–64 CrossRef CAS.
- J. Juan-Alcaniz, J. Gascon and F. Kapteijn, J. Mater. Chem., 2012, 22, 10102–10118 RSC.
- H. R. Moon, D. W. Lim and M. P. Suh, Chem. Soc. Rev., 2013, 42, 1807–1824 RSC.
- S. Opelt, S. Turk, E. Dietzsch, A. Henschel, S. Kaskel and E. Klemm, Catal. Commun., 2008, 9, 1286–1290 CrossRef CAS.
- D. Zhang, Y. Guan, E. J. M. Hensen, T. Xue and Y. Wang, Catal. Sci. Technol., 2014, 4, 795–802 CAS.
- H. Liu, L. Chang, L. Chen and Y. Li, ChemCatChem, 2016, 8, 946–951 CrossRef CAS.
- K. Manna, T. Zhang, M. Carboni, C. W. Abney and W. Lin, J. Am. Chem. Soc., 2014, 136, 13182–13185 CrossRef CAS PubMed.
- D. T. Genna, L. Y. Pfund, D. C. Samblanet, A. G. Wong-Foy, A. J. Matzger and M. S. Sanford, ACS Catal., 2016, 6, 3569–3574 CrossRef CAS.
- H. Reinsch, R. S. Pillai, R. Siegel, J. Senker, A. Lieb, G. Maurin and N. Stock, Dalton Trans., 2016, 45, 4179–4186 RSC.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Ferey, Chem.–Eur. J., 2004, 10, 1373–1382 CrossRef CAS PubMed.
- S. Sircar, Ind. Eng. Chem. Res., 2006, 45, 5435–5448 CrossRef CAS.
- D. Zhang, Y. Guan, E. J. M. Hensen, L. Chen and Y. Wang, Catal. Commun., 2013, 41, 47–51 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21701j |
| This journal is © The Royal Society of Chemistry 2016 |