One-step synthesis of hierarchical Ni–Fe–Al layered double hydroxide with excellent sensing properties for NOx at room temperature†
Dahai Honga,
Jiawei Zhangc,
Afrasiab Ur Rehmana,
Lihong Gongc,
Jiao Zhoua,
Kan Kana,
Li Li*ab and
Keying Shi*ac
aKey Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry and Material Science, Heilongjiang University, Harbin, 150080, P. R. China. E-mail: llwjjhlju@sina.cn; shikeying2008@163.com; Tel: +86 451 86609141 Tel: +86 451 86604920
bKey Laboratory of Chemical Engineering Process & Technology for High-efficiency Conversion, School of Chemistry and Material Science, Heilongjiang University, Harbin 150080, P. R. China
cKey Laboratory for Photonic and Electronic, Ministry of Education, Modern Experiment Center, Harbin Normal University, Harbin 150025, P. R. China
First published on 18th October 2016
Abstract
In this paper, hierarchical flower-like Ni–Al-layered double hydroxide (NA-LDH) and Ni–Fe–Al-layered double hydroxide (NFA-LDH) intercalation compounds were synthesized by a facile one-step hydrothermal method using sodium dodecyl sulfate (SDS) as the intercalation layer and template. The NFA-LDHs with a Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio of 1
Al molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NFA 1-1), which have porous hierarchical nanostructures and channels between the layers, provided the channels with fast carrier transportation and gas adsorption–desorption. Therefore, NFA 1-1 showed good sensing properties at 100 ppb for the detection of NOx at room temperature, and the response toward 100 ppm NOx was 82%, which was 2.3 times higher than that of pure NA-LDHs at room temperature. NFA-LDHs might be promising compounds for high performance gas sensing for commercial application. The enhanced gas sensing of NFA 1-1 can be ascribed to the unique hierarchical porous structure and the highly oriented layered single crystal structure and composition, which can enhance conductivity/carrier densities, fast carrier transportation and gas adsorption–desorption, and provide a large number of active sites for surface contact reactions.
1 (NFA 1-1), which have porous hierarchical nanostructures and channels between the layers, provided the channels with fast carrier transportation and gas adsorption–desorption. Therefore, NFA 1-1 showed good sensing properties at 100 ppb for the detection of NOx at room temperature, and the response toward 100 ppm NOx was 82%, which was 2.3 times higher than that of pure NA-LDHs at room temperature. NFA-LDHs might be promising compounds for high performance gas sensing for commercial application. The enhanced gas sensing of NFA 1-1 can be ascribed to the unique hierarchical porous structure and the highly oriented layered single crystal structure and composition, which can enhance conductivity/carrier densities, fast carrier transportation and gas adsorption–desorption, and provide a large number of active sites for surface contact reactions.
1 Introduction
NOx gas emissions (composition is NO and NO2) are one of the major environmental/air pollutants; NOx is emitted from factories, ships, automobiles, thermal power stations, and so on by the combustion of fossil fuels (coal and oil etc.).1–4 It plays a key role in the generation of acid rain and photochemical oxidants,5 and hazardous NOx gas has effects on human health and the ecosystem. Therefore, it is very important to research and develop NOx sensors to detect NOx at room temperature. At present, considerable attention has been given to hierarchical structure materials used for fabricating chemical gas sensors. For commercial application, the sensors often need a large surface area, and fast gas adsorption–desorption and diffusion,6–9 as well as needing to be low energy and low cost.10,11 Recently, there have been studies into the design and synthesis of hierarchical layered double hydroxide (LDH) materials, which possess high surface area and have exhibited excellent performance.12–17LDHs are a class of two-dimensional (2D) layered inorganic matrices.18 They can be represented by the general formula [M1−x2+Mx3+(OH)2]x+(An−)x/n·mH2O, in which M2+ and M3+ are divalent metal cations (Ni2+, Mg2+, Co2+, Zn2+ etc.) and trivalent metal cations (Fe3+, Al3+, Cr3+, Ga3+ etc.), respectively, and An− (Cl−, OH−, NO3−, SO42− etc.) is an intercalated guest anion.19,20 Generally, throughout the entire particle bulk, the double layered LDH-like structure always exhibits a gallery pathway facilitating carrier diffusion/transportation.21 As specific layered 2D structure materials, LDHs have been given considerable attention for their potential application in the fields of catalysis,22,23 drug delivery materials,24,25 electrochemistry,26,27 adsorbents for wastewater treatment28,29 and gas sensors.30,31 Moreover, LDH sensors not only work at room temperature21 with a detection limit 0.5 ppm,32 but also the range of test gas detectors is prominent, including acetone,33 ethanol34 and nitrogen oxides35 etc.
The working principle of conductive-type gas sensors is based on measuring the change in conductivity caused by gas adsorption or the process of surface reaction.36 In many instances, the response time of the gas sensor is very sensitive to the diffusion/transportation of electrons,37 because the charge carriers before reaching the surface of the unit sheet in a LDHs-like structure only have a very short (sub nanometer) distance to diffuse. So, the LDH structure is beneficial to reducing the response time compared with polycrystalline metal oxide gas sensors. Moreover, the structural characteristics of 3D hierarchical LDHs can provide a high surface area and a well-defined porosity, and can increase the sensitivity, self-diagnosis, and low-temperature operation (or short response time). Therefore, 3D LDH-like structure materials are conducive to the detection of gases, being low cost and environmentally friendly, easy to synthesize and operating at room temperature. Considering the above aspects, we are going to design and fabricate novel promising composites to improve NOx gas sensing performance.
In our previous work, Mg–Al-LDHs exhibited better sensing performance for NOx.38 However, the response to 100 ppm NOx remained constant for only 35 days at room temperature; there was almost no response and the response time was slow after 35 days. To overcome the aforementioned problem, we replaced Mg2+ with Ni2+ and Fe3+, and a simple hydrothermal method is used to prepare novel 3D hierarchical nanostructure NFA-LDH composites by adjusting the molar ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al. During the synthesis of NFA-LDHs, SDS is used as the template and intercalating agent (Scheme 1). SDS is an anionic surface active agent, and, under hydrothermal conditions and through electrostatic attraction, Ni2+, Fe3+ and Al3+ ions might form coordination complexes with sulfated groups of SDS. In comparison with NA-LDH composites, the NFA-LDH composite sensor possesses good sensitivity, the stability is three times that in our previous work, the response time was still maintained at 2–4 s, and in terms of reproducibility toward NOx at room temperature, moreover, in comparison with other NOx sensors,39–41 the NFA-LDH composite sensor possesses higher sensitivity and ultralow detection limits, which is mainly due to its distinctive 3D hierarchical flower-like structure. It is also easy to synthesise. The enhanced gas sensing performance of NFA-LDH composites successfully demonstrates their potential application as sensing materials for superior gas sensors. The hierarchical NFA-LDHs are used for the first time as NOx sensors.
Al. During the synthesis of NFA-LDHs, SDS is used as the template and intercalating agent (Scheme 1). SDS is an anionic surface active agent, and, under hydrothermal conditions and through electrostatic attraction, Ni2+, Fe3+ and Al3+ ions might form coordination complexes with sulfated groups of SDS. In comparison with NA-LDH composites, the NFA-LDH composite sensor possesses good sensitivity, the stability is three times that in our previous work, the response time was still maintained at 2–4 s, and in terms of reproducibility toward NOx at room temperature, moreover, in comparison with other NOx sensors,39–41 the NFA-LDH composite sensor possesses higher sensitivity and ultralow detection limits, which is mainly due to its distinctive 3D hierarchical flower-like structure. It is also easy to synthesise. The enhanced gas sensing performance of NFA-LDH composites successfully demonstrates their potential application as sensing materials for superior gas sensors. The hierarchical NFA-LDHs are used for the first time as NOx sensors.
2 Experimental
2.1. Synthesis of the NA-LDH composites
All of the chemicals used were analytical grade without any further purification. In a typical reaction synthesis, 0.8 mmol Ni(NO3)2·6H2O, 0.4 mmol Al(NO3)·9H2O, 0.4 mmol urea and 0.6 mmol SDS were dissolved in 40 mL distilled water and the mixed solutions were stirred continuously for 30 minutes (min). After stirring, the obtained solution was loaded into a 50 mL Teflon-lined stainless steel autoclave, sealed, and heated at 150 °C for 6 h. After cooling to room temperature, the resulting green product was washed with deionized water and ethanol several times, and then dried at 80 °C in air for 10 h and was denoted as NA-LDHs.2.2. Synthesis of the NFA-LDH composites
In a typical reaction synthesis, different amounts of Ni(NO3)2·6H2O and Fe(NO3)3·6H2O, 0.4 mmol Al(NO3)·9H2O, 0.4 mmol urea and 0.6 mmol SDS were dissolved in 40 mL distilled water, and the mixed solutions were stirred continuously for 30 min. After stirring, the obtained solution was loaded into the 50 mL Teflon-lined stainless steel autoclave, sealed, and heated at 150 °C for 6 h. After cooling to room temperature, the green product was washed (water + ethanol) several times as described above and then dried at 80 °C for 10 h to obtain NFA in air. The Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al samples with 1
Al samples with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (molar ratio) were named NFA 1-2, NFA 1-1 and NFA 2-1, respectively.
3 (molar ratio) were named NFA 1-2, NFA 1-1 and NFA 2-1, respectively.
2.3. Material characterization
The crystal phase of the composites was characterized by X-ray powder diffraction (XRD, D/max-IIIB-40 kV, Japan, Cu Ka radiation, λ = 1.5406 Å) and infrared spectroscopy (IR, Perkin Elmer Spectrometer, KBr pellet technique). The morphologies and structures of the composites were characterised using scanning electron microscopy (SEM, Hitachi S-4800) and transmission electron microscopy (TEM, JEOL 2100) at an accelerating voltage of 200 kV. The Brunauer–Emmett–Teller (BET) surface area of the products was measured by N2 adsorption–desorption (Quantachrome Instruments, USA). The samples were dried for 10 h at 150 °C under vacuum before the measurements. Electrochemical impedance spectroscopy (EIS) and Mott–Schottky (MS) plot measurements were carried out using an electrochemical working station (CHI660C, Shanghai, China) in a half-cell setup configuration at room temperature. In the EIS measurements, the range of frequency was 0.01 Hz to 100 kHz and the excitation amplitude was 0.4 V.2.4. Measurement of gas sensors
5.0 mg of sample was ultrasonically dispersed in 0.1 mL deionized water. 0.05 mL of the suspension was dropped onto an interdigitated Au electrode (7.0 × 5.0 × 0.38 mm), and then dried at 60 °C for 4 h to obtain a film device. The thickness of the film was about 0.03 to 0.04 mm. The distance of the two neighboring Au fingers was about 20 μm (each Au electrode contained 100 fingers). The Au electrode on the Al2O3 substrate was cleaned using diluted HCl, distilled water and acetone. The sensors were dried at 60 °C for 5 h and fabricated by a drop-casting method. The sensor was tested in an airtight glass chamber at room temperature and relative humidity (RH) of about 26%. The gas concentration was controlled by injecting a specific volume of gas and the chamber was purged with air to recover the sensor resistance. The sensor sensitivity (S) values were calculated according to the following formula:| S = |Rg − Ra|/Ra × 100% (Ra > Rg) |
3 Results and discussion
3.1. Structural and morphological characteristics
XRD is a powerful and essential apparatus for evaluating the structure of materials. Fig. 1a displays typical XRD patterns for the samples. For all samples, the peaks at 2θ values of 11.7°, 23.6°, 35.1° and 61.2° correspond to the (003), (006), (012) and (110) planes of a typical LDH. Moreover, no secondary crystalline phases were found in the NA-LDHs or NFA-LDHs, and all the diffraction peaks can be indexed to a rhombohedral structure (LDHs) (JCPDS no. 15-0087).It can be seen that the NFA-LDH composites show similar XRD patterns to those of the NA-LDHs. This indicates that the presence of Fe3+ does not influence the crystal structure of the synthesized NA-LDHs. Furthermore, the XRD patterns of samples with added Fe(OH)3 show a decreased intensity of this (003) peak, which demonstrates that the Fe3+ has been doped on the NA-LDHs and corresponds to the lower crystallinity of the LDHs.
Fig. 1b shows the IR spectra of NA-LDHs and NFA-LDHs. We can observe that a broad absorption at 3438 cm−1 corresponds to the O–H stretching vibrations of hydroxyl groups of interlayer water molecules and brucite layers. The asymmetric and symmetric stretching modes of C–H correspond to 2923 and 2853 cm−1 respectively. The weaker peak at 1630 cm−1 is the bending mode of water molecules. The peaks at 1380 and 1064 cm−1 were assigned to the asymmetric and symmetric stretching modes of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Meanwhile, some of the bands below 800 cm−1 are due to the characteristic bending vibrations of metal–oxygen (M–O) bonds, confirming the existence of NFA-LDHs.
O. Meanwhile, some of the bands below 800 cm−1 are due to the characteristic bending vibrations of metal–oxygen (M–O) bonds, confirming the existence of NFA-LDHs.
The general morphology of the prepared NA-LDH and NFA-LDH materials was investigated by SEM and TEM, and the results are displayed in Fig. 2 and S1.† The morphology of NFA 1-2, NFA 1-1 and NFA 2-1, which formed aggregated nanosheets of irregular shape, is confirmed by the SEM. The prepared samples of NFA 1-2, NFA 1-1 and NFA 2-1 possess 3D hierarchical flower-like structures. A large number of pores exist between the nanosheets and the 3D structure. The thin nanosheet structures consist of [M1−x2+Mx3+(OH)2]x+ layers, with many inter-spaces and channels between the layers. It can be seen in Fig. 2A and B that the NFA 1-2 composites do not possesses excellent 3D hierarchical structure, and for NFA 1-1 in Fig. 2C and D, the thin nanosheet density is much higher than that of NFA 1-2 and NFA 2-1, with better dispersion and 3D hierarchical structures. From this it can be deduced that an increase in the contact area allows better permeability between the hierarchical NFA 1-1 and gas molecules.
The microstructure and morphology of NFA 1-1 were observed by TEM and HRTEM and 3D hierarchical microstructures of NFA 1-1 are depicted in Fig. 3, S2 and S3.† The low magnification TEM images (Fig. 3A-a, and S2a†) display the 3D hierarchical flower-like structures of the NFA 1-1 nanosheet. It can be observed that these architectures together were indeed distinguishable nanopetals/nanosheets, which were similar to the SEM results in Fig. 2C and D. Magnification of the nanopetals, shown in Fig. 3b–e, S2b and S3,† revealed that the nanopetals consist of porous thin nanosheets, and 4–8 layers of nanosheets were found. Moreover, this explains the presence of 2–3 nm mesopores on the nanopetals and nanosheets. These mesopores were formed by exuberant release of CO2 caused by the urea hydrolysis process, and may enhance the gas sensing properties. HRTEM images of NFA 1-1 are shown in Fig. 3f and S2c.† We can clearly see the lattice fringes and observe fringe spacings of 0.19 and 0.25 nm, which are in agreement with the (018) and (012) crystal planes of NFA 1-1, respectively. The SAED pattern is inserted in the lower left corner of Fig. 3b. The polycrystalline diffraction rings from the inside to outside correspond to the (012), (018), and (110) planes (Fig. 3b) of cubic phase NFA-LDHs, which is in agreement with the XRD results. Finally, a study was carried out on more than twenty nanopetals and the results reveal that the nanopetals were porous single crystals.
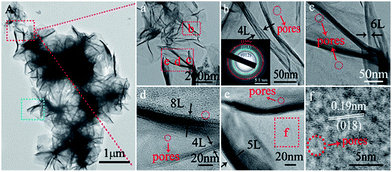 | ||
| Fig. 3 TEM images of NFA 1-1 (A); (a) low magnification TEM image; (b–e) TEM images of (a), the inset in (b) is a selected-area electron diffraction (SAED) pattern; (f) HRTEM image of (e). | ||
The N2 adsorption–desorption isotherms of all the samples were measured as shown in Fig. S4.† A type-IV isotherm with a H3-type hysteresis loop can be clearly seen. The BET surface areas of NA-LDH, NFA 1-2, NFA 1-1 and NFA 2-1 were found to be 127.5, 181.6, 192.9 and 181.4 m2 g−1 respectively (see Fig. S4†). Moreover, the inset of Fig. S4a–d† shows the pore size distribution curves of NA-LDH, NFA 1-2, NFA 1-1 and NFA 2-1 respectively, and the mesopore distribution of all samples is almost at 3.93 nm. The NFA 1-1 sample showed a higher specific surface area and the dominant type of pore was the mesopore, which means that the adsorption–diffusion of gas is much easier and this is greatly advantageous in improving the sensitivity of the gas sensor.
Fig. S5† shows the STEM image/EDS mapping of the NFA 1-1 sample. The bright field images of NFA 1-1 shown in Fig. S5a and b† are EDS spectra, and Fig. S5c–f† respectively show a uniform distribution of four elements (Ni, Al, Fe and O) within the sample.
The results of the SEM and TEM illustrate the growth mechanism for the hierarchical NFA-LDH nanostructures discussed and described in Scheme 2.
 | ||
| Scheme 2 Schematic illustration of the formation mechanism of hierarchical NFA-LDHs by a hydrothermal method at 150 °C for 6 hours. | ||
Since SDS is an anionic surface active agent, under hydrothermal conditions and through electrostatic attraction, Ni2+, Fe3+ and Al3+ ions might form coordination complexes with the sulfated groups of SDS. Then, urea can be hydrolyzed gradually (eqn (1) shows the generation of NH4+, CO2, and OH−), and an alkaline solution is obtained. Consequently, LDH crystals formed due to the precipitation of Ni2+ (Fe3+ and Al3+) ions, which provides the necessary heterogeneous nucleation sites. The structures were based on the sharing of Ni(OH)64− octahedral unit edges to build M(OH)2 brucite-like layers,42 in which some of the divalent cations (Ni2+) were replaced by trivalent cations (Fe3+ and Al3+) and the lattices were positively charged. Sequentially, positively charged lattices of NFA-LDH crystals will be attached to the negatively charged sulfated groups, finally gradually building up (eqn (2)).
| CO(NH2)2 + 3H2O → 2NH4+ + CO2↑ + OH− | (1) |
| Ni2 + (DS−) + Fe3 + (DS−) + Al3 + (DS−) + 2OH− + mH2O → NiFeAl(OH)2(DS)n·mH2O | (2) |
Here, it can be speculated that, due to the nanosheet structure of the NFA-LDHs crystals and through the bending force, the NFA-LDH crystal layers can be deformed, and the bending force originates from the coordination sites of the SDS molecules with metal ions, resulting in the curved nanosheets.43 Then, the curved or contorted nanosheets self-assemble into 3D flower-like nanostructures.
3.2. Gas sensing properties
The NOx gas sensing properties of 3D hierarchical NFA-LDH sensors were investigated at room temperature in air. Fig. 4a shows the relationship between sensitivity and response time of different concentrations of NOx for NFA-LDHs. For the NFA 1-1 sensor, when the concentration of NOx was 100 ppm, the response time was only 2.6 s, and the sensitivity reached 82%, which was two times faster than pure NA-LDHs for 100 ppm NOx. In particular, it is worth noting that the NOx detection limit of NFA 1-1, at the lowest limit of detection, dropped to 0.1 ppm NOx and the response reached 18%. Compared with other NOx sensors, NFA 1-1 sensors not only show high sensitivity, but also show a very low detection limit.30,38The dynamic response recovery curves of NA-LDHs, NFA 2-1, NFA 1-1 and NFA 1-2 thin film sensors to NOx at room temperature are shown in Fig. 4c and d and S6.† Obviously, the resistance declines drastically when NOx gas is injected, and then rises to its initial position when the sensors are exposed to air for some time, indicating a typical gas response between the p-type semiconductor and the NOx gas. It can be observed that the thin-film sensor based on NFA 1-1 exhibits a rapid and reversible response signal to NOx gas even at the lowest exposure level (0.1 ppm) in both the adsorption and desorption processes. With an increase of the concentration, the response time is also shortened (Fig. 4b). The sensitivity results of the NFA 1-1 sensor to NOx at room temperature in air is shown in Table S1.†
From Fig. 4c, it can be seen that the sensitivity of the four composites declined when the concentration of NOx decreased from 100 ppm to 0.1 ppm. Especially for NFA 1-1, the detection limit was lower compared with NFA 2-1, and NFA 1-1 showed better sensing performance compared with NFA 1-2, while the lowest detectable NOx concentration of NA-LDHs was only 3 ppm. In addition, the response times to 100–0.1 ppm NOx at room temperature for the four composites in Fig. 4d obviously show that the response time of NFA 1-1 was the fastest among the composites.
In order to study the selectivity of the NFA 1-1 sensor, the selectivities to some testing gases including H2, CO, NH3 and CH4 at room temperature were measured and are shown in Fig. 4e. NOx measurements were carried out at 100 ppm, for which the sensitivity reached 82%, but other gases were measured at 1000 ppm and the sensitivity to H2, NH3, CH4, SO2, CO2 and C2H6O only reached 3.7%, 4.7%, 4.4%, 6%, 1.1% and 2.9% respectively, and almost no sensitivity to CO was observed. Obviously, the NFA 1-1 sensor exhibited excellent selectivity to NOx. The stability of the NFA 1-1 sensor was measured at 100 ppm NOx for 106 days as shown in Fig. 4f, and the response time remained at 2–4.2 s to 100 ppm for 106 days at room temperature, which demonstrates favorable stability.
In order to examine the influence of the Fe3+ ion replacement property (in NFA-LDHs) on the gas sensing properties, the carrier concentrations of NA-LDH, NFA 2-1, NFA 1-1 and NFA 1-2 composites were measured using a MS method, and the results are shown in Fig. 5A. The MS curves of NA-LDH, NFA 2-1, NFA 1-1 and NFA 1-2 composites were measured using the MS method, and the results show a negative slope in the MS plots and p-type semiconducting behavior. The lower the slope in the MS plot, the higher the carrier concentration. The carrier densities of NA-LDH, NFA 2-1, NFA 1-1 and NFA 1-2 are 1.05 × 1018, 4.15 × 1018, 4.9 × 1018 and 1.87 × 1018 respectively. The carrier densities were calculated using eqn (1).
| (1/C2)/V = −2/(qε0εrND) |
As we know that the resistance of materials in air plays a key role in the gas sensing response, a suitable resistance and better electron transportation would induce shorter response time. Thus, EIS measurements to study the charge transfer rate reveal the expected Nyquist plots for the samples, as shown in Fig. 5B. The Nyquist plots were fitted using an appropriate electric equivalent circuit (the inset of Fig. 5B) and impedance parameters were obtained as given in Table S2.† Here RΩ indicates the uncompensated bulk resistance of the electrode, electrolyte and separator, Rct is attributed to the charge-transfer resistance at the active material interface and C is the constant phase angle element, involving double layer capacitance. A straight sloping line represents diffusive resistance of proton diffusion in the host materials and electrolyte in the electrode pores. The semicircle in the high-frequency range is associated with the surface properties of the porous electrode, which correspond to Rct. Both RΩ and Rct are related to the conductivity of the electrodes. The results show that the values of uncompensated bulk resistance RΩ and charge-transfer resistance Rct were 60.91 and 329.2 Ω for NFA 1-1, which are significantly lower than those of NA-LDHs (see Table S2†). Therefore, some Fe3+ ion replacement of Ni2+ (or Al3+) in NA-LDHs could enhance the adsorption of electrons and transportation from the gas sensor on the surface, so that electrons can easily transfer from the NFA-LDH surface to O2 (in air) or NOx. Therefore, NFA 1-1 possesses a highly oriented layered polycrystalline structure, which improves electron transportation from the NFA 1-1 sensor to the surface adsorbates NOx.
3.3. Gas sensing mechanism of NFA-LDHs
NFA-LDHs have porous hierarchical nanostructures and channels between the layers, which provide channels/gallery pathways with fast carrier transportation and gas adsorption–desorption. The enhanced gas sensing properties of NFA 1-1 at room temperature are a result of the following two factors. The first reason can be attributed to the synergistic effects of the 3D hierarchical LDHs structures. NOx is a kind of strong oxidizer. Charge transfer is likely to occur from the NFA-LDHs to NOx as governed by reactions (R2) and (R3). This means that when the sensor film is in the oxidizing gas NOx, the gas molecules could attract the electrons from the NFA-LDH nanostructures, due to NOx molecules with high electron affinity, which leads to electron transfer from the NFA-LDH layers to the adsorbates NOx. Also the 3D hierarchical LDH structures provide a lot of mesopores/micropores and good permeability for hierarchical NFA 1-1, which are conducive to gas diffusion, adsorption/desorption and provide a large number of active sites for surface contact reactions. Meanwhile, rapid and effective NOx gas diffusion towards both the surface and the inner region of the hierarchical LDH-like nanostructures can be accomplished easily. Thus, NOx molecules are adsorbed on the NFA 1-1 gas sensor which not only has a fast response time, but also has ultralow detection limits.Secondly, the excellent sensing properties of NFA 1-1 may be attributed to the highly oriented layered single crystal structure and composition. Some Fe3+ ion replacement of Ni2+ (or Al3+) in NA-LDHs can enhance the carrier densities of NFA 1-1, which might improve the adsorption of electrons and transportation from the sensor on the surface, so that electrons can easily be transferred from the NFA-LDH layered surface to the surface adsorbates O2 or NOx (Scheme 3).
In addition, the LDH-like structure with enlarged interlayer spacing (∼0.754 nm) by DS− anions has always exhibited a gallery pathway promoting carrier diffusion/transportation throughout the entire flower like layers, and charge carriers only need a very short (sub-nanometre) distance to diffuse before reaching the surface of the unit sheet. These rapid hole-trapping processes allow electrons to diffuse sufficiently and freely within unit sheets until reaching the sheet edges. Meanwhile, NFA-LDHs may provide a large number of hydroxyl groups, and more hydrogen bonds between surface hydroxyl groups and interlayer water molecules, equivalent to a bridge connecting the adjacent NFA-LDH layers. This means that the bridging effect can provide more natural channels for the effective and fast transportation of carriers. Moreover, interlayer water oxygen atoms are electron acceptor atoms, so sulfated groups and water molecules will act as an electron gallery pathway. Thus, the NFA 1-1 sensor exhibits a greater response and a fast response time. The gas sensing reactions are given below:
| O2 + e− ↔ O2−(2O−) | (R1) |
| NOx + e → NOx− | (R2) |
| NOx + O2− + 2e → NOx+1− + O− | (R3) |
4 Conclusions
The hierarchical flower-like NFA-LDHs have been synthesized using a low cost and facile hydrothermal method. The Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al samples with 1
Al samples with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (molar ratio) were named NFA 1-2, NFA 1-1 and NFA 2-1, respectively. The synthesized NFA-LDHs with a Ni
3 (molar ratio) were named NFA 1-2, NFA 1-1 and NFA 2-1, respectively. The synthesized NFA-LDHs with a Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio of 1
Al molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed excellent sensing properties for NOx gas with a low detection concentration of 0.1 ppm, high sensitivity which can reach 82%, a fast response time of 2.6 s at 100 ppm, and the response time remained at 2–4.2 s for 100 ppm for 106 days at room temperature. The enhanced gas sensing of NFA 1-1 can be ascribed to the unique hierarchical porous structure and the highly oriented layered single crystal structure and composition, which can enhance the conductivity/carrier densities, fast carrier transportation, gas adsorption–desorption, and provide large numbers of active sites for surface contact reaction. As described above the nanopetals consist of thin porous nanosheets, and 4–8 layer of nanosheets were found. Moreover, this explains the presence of 2–3 nm mesopores on the nanopetals and nanosheets. These mesopores were formed by exuberant release of CO2 caused by the urea hydrolysis process, and this enhances the gas sensing property. NA-LDHs have promising applications for the improvement of gas sensing performance of NiAl-based gas sensors, particularly for NOx.
1 showed excellent sensing properties for NOx gas with a low detection concentration of 0.1 ppm, high sensitivity which can reach 82%, a fast response time of 2.6 s at 100 ppm, and the response time remained at 2–4.2 s for 100 ppm for 106 days at room temperature. The enhanced gas sensing of NFA 1-1 can be ascribed to the unique hierarchical porous structure and the highly oriented layered single crystal structure and composition, which can enhance the conductivity/carrier densities, fast carrier transportation, gas adsorption–desorption, and provide large numbers of active sites for surface contact reaction. As described above the nanopetals consist of thin porous nanosheets, and 4–8 layer of nanosheets were found. Moreover, this explains the presence of 2–3 nm mesopores on the nanopetals and nanosheets. These mesopores were formed by exuberant release of CO2 caused by the urea hydrolysis process, and this enhances the gas sensing property. NA-LDHs have promising applications for the improvement of gas sensing performance of NiAl-based gas sensors, particularly for NOx.
Acknowledgements
This work was supported by the Program for Innovative Research Team in Chinese Universities (IRT1237); the National Natural Science Foundation of China (No. 2167010747); the National Natural Science Foundation of Heilongjiang province (No. 2015003), the Program for Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education in Heilongjiang University and Harbin Normal University (PEBM201502).References
- J. Z. Bloh, A. Folli and D. E. Macphee, RSC Adv., 2014, 4, 45726–45734 RSC.
- X. Gao, Z. Du, H.-L. Ding, Z.-L. Wu, H. Lu, Z.-Y. Luo and K.-F. Cen, Energy Fuels, 2010, 24, 5876–5882 CrossRef CAS.
- H. Wang, Q. Q. Yu, T. Liu, L. P. Xiao and X. M. Zheng, RSC Adv., 2012, 2, 5094–5097 RSC.
- J. Wang, X. Li, Y. Xia, S. Komarneni, H. Y. Chen, J. L. Xu, L. Xiang and D. Xie, ACS Appl. Mater. Interfaces, 2016, 8, 8600–8607 CAS.
- A. C. O. Plens, D. L. G. Monaro and A. R. Coutinho, An. Acad. Bras. Cienc., 2015, 87, 1149–1160 CrossRef PubMed.
- L. W. Wang, Y. H. Wang, K. F. Yu, S. P. Wang, Y. Y. Zhang and C. H. Wei, Sens. Actuators, B, 2016, 232, 91–101 CrossRef CAS.
- Y. Lin, C. Li, W. Wei, Y. J. Li, S. P. Wen, D. M. Sun, Y. Chen and S. P. Ruan, RSC Adv., 2015, 5, 61521–61527 RSC.
- Z. Z. Pan, F. Q. Sun, S. P. Xu, J. F. Long, Y. Chen and Z. F. Zhuang, RSC Adv., 2015, 5, 74075–74083 RSC.
- Y. J. Li, X. H. Ma, S. J. Guo, B. Wang, D. M. Sun, X. D. Zhang and S. P. Ruan, RSC Adv., 2016, 6, 22889–22895 RSC.
- C. H. Zheng, T. Yao, T. R. Xu, H. A. Wang, P. F. Huang, Y. Yan and D. L. Fang, J. Alloys Compd., 2016, 678, 93–101 CrossRef CAS.
- Y. P. Zhang, H. P. Li, N. Du, R. J. Zhang and W. G. Hou, Colloids Surf., A, 2016, 501, 49–54 CrossRef CAS.
- T. Li, G. H. Li, L. H. Li, L. Liu, Y. Xu, H. Y. Ding and T. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 2562–2572 CAS.
- S. X. Cai, D. S. Zhang, L. Y. Shi, J. Xu, L. Zhang, L. Huang, H. R. Li and J. P. Zhang, Nanoscale, 2014, 6, 7346–7353 RSC.
- L. H. Yao, D. Wei, D. P. Yan and C. G. Hu, Chem.–Asian J., 2015, 10, 630–636 CrossRef CAS PubMed.
- X. J. Du, D. S. Zhang, L. Y. Shi, R. H. Gao and J. P. Zhang, Nanoscale, 2013, 5, 2659–2663 RSC.
- X. J. Du, D. S. Zhang, R. H. Gao, L. Huang, L. Y. Shi and J. P. Zhang, Chem. Commun., 2013, 49, 6770–6772 RSC.
- H. R. Li, D. S. Zhang, P. Maitarad, L. Y. Shi, R. H. Gao, J. P. Zhang and W. G. Cao, Chem. Commun., 2012, 48, 10645–10647 RSC.
- F. A. Rad, Z. Rezvani and F. Khodam, RSC Adv., 2016, 6, 11193–11203 RSC.
- Q.-H. Xu, D.-M. Xu, M.-Y. Guan, Y. Guo, Q. Qi and G.-D. Li, Sens. Actuators, B, 2013, 177, 1134–1141 CrossRef CAS.
- Y. H. Ao, D. D. Wang, P. F. Wang, C. Wang, J. Hou and J. Qian, RSC Adv., 2015, 5, 54613–54621 RSC.
- D. M. Xu, M. Y. Guan, Q. H. Xu and Y. Guo, J. Hazard. Mater., 2013, 262, 64–70 CrossRef CAS PubMed.
- Y. P. Zeng, T. C. Zhang, Y. Y. Xu, T. Ye, R. R. Wang, Z. W. Yang, Z. H. Jia and S. G. Ju, Appl. Clay Sci., 2016, 126, 207–214 CrossRef CAS.
- T. P. Sulmonetti, S. H. Pang, M. T. Claure, S. Lee, D. A. Cullen, P. K. Agrawal and C. W. Jones, Appl. Catal., A, 2016, 517, 187–195 CrossRef CAS.
- M. A. Rocha, P. A. D. Petersen, E. Teixeira-Neto, H. M. Petrilli, F. Leroux, C. T. Gueho and V. R. L. Constantino, RSC Adv., 2016, 6, 16419–16436 RSC.
- S. Y. Guan, R. Z. Liang, C. Y. Li, D. Yan, M. Wei, D. G. Evans and X. Duan, J. Mater. Chem. B, 2016, 4, 1331–1336 RSC.
- J. P. Cheng, J. Zhang and F. Liu, RSC Adv., 2014, 4, 38893–38917 RSC.
- J. Memon, J. H. Sun, D. L. Meng, W. Z. Ouyang, M. A. Memon, Y. Huang, S. K. Yan and J. X. Geng, J. Mater. Chem. A, 2014, 2, 5060–5067 CAS.
- Y. Lin, Z. K. Zeng, J. R. Zhu, Y. Wei, S. Chen, X. Yuan and L. Liu, Mater. Lett., 2015, 156, 169–172 CrossRef CAS.
- G. Darmograi, B. Prelot, G. Layrac, D. Tichit, G. M. Gassin, F. Salles and J. Zajac, J. Phys. Chem. C, 2015, 119, 23388–23397 CAS.
- Y. L. Ge, K. Kan, Y. Yang, L. Zhou, L. Q Jing, P. K. Shen, L. Li and K. Y. Shi, J. Mater. Chem. A, 2014, 2, 4961–4969 CAS.
- Y. Guo, Y. P. Xiao, L. M. Zhang and Y.-F. Song, Ind. Eng. Chem. Res., 2012, 51, 8966–8973 CrossRef CAS.
- Z. Y. Chu, H. X. Sun, H. Xu, J. Zhou, G. Zhang, Y. Xie, L. Li and K. Y. Shi, RSC Adv., 2015, 5, 101760–101767 RSC.
- L. J. Zhang, W. Q. Rong, Y. C. Chen, C. Lu and L. X. Zhao, Sens. Actuators, B, 2014, 205, 82–87 CrossRef CAS.
- M. Y Guan, D. M Xu, Y. F. Song and Y. Guo, Sens. Actuators, B, 2013, 188, 1148–1154 CrossRef.
- H. J. Wang, J. Gao, Z. G. Li, Y. L. Ge, K. Kan and K. Y. Shi, CrystEngComm, 2012, 14, 6843–6852 RSC.
- C. Wang, X. Y. Cheng, X. Zhou, P. Sun, X. L. Hu, K. Shimanoe, G. Y. Lu and N. Yamazoe, ACS Appl. Mater. Interfaces, 2014, 6, 12031–12037 CAS.
- J. C. Wang, L. L. Wang, J. Gao, L. Zhou, Y. L. Ge, L. Q. Jing, K. Y. Shi and L. Li, Mater. Electron., 2015, 26, 6612–6624 CrossRef CAS.
- H. X. Sun, Z. Y. Chu, D. H. Hong, G. Zhang, Y. Xie, L. Li and K. Y. Shi, J. Alloys Compd., 2016, 658, 561–568 CrossRef CAS.
- W. Z. Song, H. Y. Wu, J. C. Wang, Y. F. Lin, J. B. Song, Y. Xie, L. Li and K. Y. Shi, Aust. J. Chem., 2015, 68, 1569–1576 CrossRef CAS.
- Y. Yang, L. Sun, X. T. Dong, H. Yu, T. T. Wang, J. X. Wang, R. H. Wang, W. S. Yua and G. X. Liu, RSC Adv., 2016, 6, 37085–37092 RSC.
- J. Gao, H. Y. Wu, J. Zhou, L. Y. Yao, G. Zhang, S. Xu, Y. Xie, L. Li and K. Y. Shi, New J. Chem., 2016, 40, 1306–1311 RSC.
- G. Abellan, C. Martí-Gastaldo, A. Ribera and E. Coronado, Acc. Chem. Res., 2015, 48, 1601–1611 CrossRef CAS PubMed.
- H. Wang, G. L. Fan, C. Zheng, X. Xiang and F. Li, Ind. Eng. Chem. Res., 2010, 49, 2759–2767 CrossRef CAS.
- Y. Yang, H. J. Wang, L. L. Wang, Y. L. Ge, K. Kan, K. Y. Shi and J. H. Chen, New J. Chem., 2016, 40, 4678–4686 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21645e |
| This journal is © The Royal Society of Chemistry 2016 |






