Conductive casting films based on an octasilicate-core dendrimer containing the mixed-valence state TCNQ on the periphery†
Yasuyuki Iriea,
Lina Lia,
Hiroaki Imotoa,
Megumi Komadab,
Takashi Nishinob and
Kensuke Naka*a
aFaculty of Molecular Chemistry and Engineering, Graduate School of Science and Technology, Kyoto Institute of Technology, Goshokaido-cho, Matsugasaki, Sakyo-ku, Kyoto 606-8585, Japan. E-mail: kenaka@kit.ac.jp
bGraduate School of Engineering, Kobe University, Japan
First published on 2nd December 2016
Abstract
An octasilicate (OS)-core dendrimer terminated with imidazolium–7,7,8,8-tetracyanoquiodimethane (TCNQ) anion radicals (OS-mimTCNQ) was synthesized from an OS-core dendrimer having alkyl bromides as terminal groups (OS-mimBr) via anion exchange reaction with lithium TCNQ salt (LiTCNQ) in water. Conductive films were prepared by casting mixtures of OS-mimTCNQ with the neutral TCNQ in acetonitrile on glass substrates. Although the casting film of OS-mimTCNQ showed low conductivity (<10−6 S cm−1), the casting films showed relatively high electron conductivity. In particular, the casting film with the same feed molar ratio between the neutral TCNQ and the terminated imidazolium moiety (OS-CT1.0) showed the highest conductivity (5.4 × 10−2 S cm−1). The stacking structure with the mixed-valence state of TCNQ in these casting films was confirmed by UV-vis-NIR spectra. The grazing incidence X-ray diffraction (GI-XD) of OS-CT1.0 showed an anisotropic pattern, indicating that the OS-core dendrimer arranged in the direction of the out-of-plane.
Introduction
7,7,8,8-Tetracyanoquinodimethane (TCNQ) has been utilized as an electron acceptor, and it is well known that it forms charge transfer (CT) complexes with electron donor molecules. In the crystalline state, both the molecules form segregate stacks with mixed-valence state to show high electron conductivity.1 Thus, the CT complexes composed of TCNQ have attracted much attention toward electronic applications, e.g., conductors, semiconductors, and superconductive materials.2 TCNQ can form anion radical species with various metal and organic counter cations.3 The TCNQ anion radicals work as an electron donor, and consequently the mixture of neutral TCNQ and TCNQ anion radicals can construct conductive stacking structures with mixed-valence state.4,5 On the other hand, their brittleness derived from the crystalline character prohibits their practical applications.6 To overcome this problem, polymeric cations such as ionenes and poly(N-methylvinylpyridinium bromide) are applied for the formation of CT complexes.7 After the counter anions of these polymeric cations are replaced by TCNQ anion radicals, conductive films are produced by casting with the neutral TCNQ. However, formation of continuous conducting pathway derived from the TCNQ in a mixed-valence state is hardly achieved because of their random coil structures, which lead to a decrease their electron conductivities (≤10−3 S cm−1). Therefore, development of new polymeric scaffolds to control the molecular arrangement is desired to improve the material properties of conductive CT materials.Polyhedral oligomeric silsesquioxanes (POSSs) of the formula R8Si8O12, and an octasilicate (OS) of the formula (Si8O20)8− have attracted much attention because their polyhedral inorganic cores can inhibit molecular motion of their organic branches and arrange the branches in a radial fashion, leading to form the globular architectures covered with terminal groups.8 Thanks to their rigid inorganic cores, POSS- and OS-core dendrimers contribute improvement of mechanical and thermal properties. Because of these aspects, the POSS- and OS-core dendrimers terminated with functional units are ideal candidates for the construction of solid materials which are well designed in nano-level.9 Recently, we have reported single component free-standing films based on the OS-core dendrimers bearing 16 aromatic groups as the peripheral units.10 The OS-core dendrimers are suitable for the study of solid state dendrimer materials due to their film-formability.
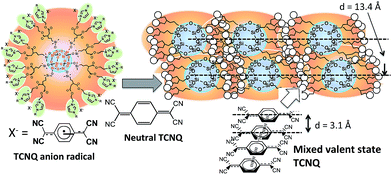
In this work, we synthesized an OS-core dendrimer having imidazolium cations as terminal groups, of which counter cations are TCNQ anion radicals (OS-mimTCNQ). The TCNQs in a mixed-valence state were prepared on the peripheries of the OS-core dendrimer by the addition of the neutral TCNQ, and conductive casting films were fabricated on substrates (Fig. 1). The casting films with several tens of micron thickness showed relatively high electron conductivity. We found that the casting film with the same feed molar ratio between the neutral TCNQ and the terminated imidazolium moiety showed the highest conductivity.
Experimental
Materials
All solvents and chemicals were of reagent-grade quality and used without further purification. 1-Methyl-3-n-propylimidazolium bromide (PmimBr),11 and octakis(propenylsuccinicanhydrido)octasilicate (OS-SA)12 were prepared using the methods in the literatures.Measurement
1H (400 MHz), 13C (100 MHz) and 29Si (80 MHz) nuclear magnetic resonance (NMR) spectra were recorded on a Bruker DPX-400 spectrometer (Bruker Biospin GmbH, Rheinstetten, Germany). Ultraviolet-visible (UV-vis) spectra were recorded on a Jasco spectrophotometer V-670 KKN (Jasco, Tokyo, Japan). Fourier transform infrared spectra (FT-IR) were obtained on a JASCO FT/IR-4100 spectrometer (JASCO, Tokyo, Japan) using KBr pellets. Matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was conducted on a Bruker Autoflex II instrument (Bruker Daltonics, Billerica, MA, USA) using dithranol matrix. The morphologies of the casting films were observed using an EV-8800 scanning electron microscope (SEM) (KEYENCE, Osaka, Japan) and a JSM-7600F field emission SEM (FESEM) (JEOL Ltd, Tokyo, Japan) with X-ray spectrometry (EDX) of X-max (OXFORD Instruments, Oxfordshire, UK). Electrical conductivity of the casting samples was measured by a four-probe technique at room temperature using Loresta-EP MCP-T360 (Mitsubishi Chemical Co.). Powder X-ray diffractometry (XRD) studies were performed on a Rigaku Smartlab X-ray diffractometer with Cu Kα radiation (λ = 1.5406 Å) in the 2θ/θ mode at room temperature. The 2θ scan data were collected at 0.01° intervals and the scan speed was 5° (2θ)/min. The grazing incidence X-ray diffraction (GI-XD) was carried out on RIGAKU X-ray Diffractometer SmartLab with in-plane and out-of-plane geometries (CuKα, 40 kV/30 mA, incidence angle α: 0.15°, 2θ: 3° to 40°).Lithium TCNQ salt (LiTCNQ)
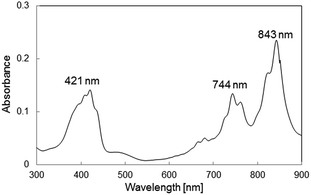
By using the method of the literature,13 lithium TCNQ salt (LiTCNQ) was prepared by reaction of TCNQ with lithium iodide. TCNQ (0.504 g, 2.47 mmol) was dissolved in acetonitrile (50 ml) at 40 °C under N2. To this solution, lithium iodide (1.00 g, 7.50 mmol) in acetonitrile (3.0 ml) was added. After stirring the mixture under reflux condition for 2 h, the residue was washed with diethyl ether (5 ml × 4 times). The yield of LiTCNQ was 0.498 g (96%). UV λmax (acetonitrile): 421, 744, 843 nm. FT-IR (KBr): υ = 2208, 2194, 2180, 1509, 826 cm−1.Bromoethyl-terminated OS-core dendrimer (OS-Br)
OS-SA (0.50 g, 0.23 mmol), 2-bromoethanol (0.14 ml, 1.98 mmol), and a catalytic amount of 4-dimethylaminopyridine (DMAP) (25.0 mg, 0.21 mmol) were dissolved in 1,4-dioxane (30 ml). The reaction was allowed to proceed for 12 h at 70 °C, then concentrated by rotary evaporator at 40 °C and dried under vacuum at ambient temperature. The residue was further dissolved in dichloromethane (30 ml). To this solution, 2-bromoethanol (0.14 ml, 1.98 mmol), DMAP (30.0 mg, 0.27 mmol), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC) (0.81 g, 4.21 mmol) were added. After stirring the mixture at room temperature under N2 for 16 h, the reaction mixture was purified by separating funnel with 1 M HCl, aqueous saturated NaHCO3, brine, then dried over anhydrous MgSO4, and evaporated under vacuum. The residue was dissolved in CHCl3 and purified by selective precipitation from methanol. The yield of pure OS-Br was 0.50 g (53%). 1H-NMR (CDCl3): δ 0.055 (s, 6H, –SiC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 0.54 (m, 2H, –SiC
3), 0.54 (m, 2H, –SiC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), 1.29 (quint, 2H, –SiCH2C
2–), 1.29 (quint, 2H, –SiCH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), 1.49 (m, 1H, –SiCH2CH2C
2–), 1.49 (m, 1H, –SiCH2CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –), 1.63 (sext, 1H, –SiCH2CH2C
–), 1.63 (sext, 1H, –SiCH2CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –), 2.44–2.68 (m, 2H, –OCOC
–), 2.44–2.68 (m, 2H, –OCOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), 2.81 (m, 1H, –OCOC
2–), 2.81 (m, 1H, –OCOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –), 3.45 (m, 4H, –COOC
–), 3.45 (m, 4H, –COOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), 4.31 (m, 4H, –COOCH2C
2–), 4.31 (m, 4H, –COOCH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–) ppm. 13C-NMR (CDCl3): δ 0.0005, 17.73, 20.77, 29.03, 29.18, 35.68, 35.94, 41.12, 64.31, 171.62, 174.49 ppm. 29Si-NMR (CDCl3): δ 12.74
2–) ppm. 13C-NMR (CDCl3): δ 0.0005, 17.73, 20.77, 29.03, 29.18, 35.68, 35.94, 41.12, 64.31, 171.62, 174.49 ppm. 29Si-NMR (CDCl3): δ 12.74  , −107.49
, −107.49  ppm. MALDI-TOF MS (m/z): calcd for C104H184Br16O52Si16K [M + K]+: 4033.5; found: 4034.0.
ppm. MALDI-TOF MS (m/z): calcd for C104H184Br16O52Si16K [M + K]+: 4033.5; found: 4034.0.
Imidazolium bromide terminated OS-core dendrimer (OS-mimBr)
OS-Br (1.00 g, 0.25 mmol) was dissolved in 1-methylimidazole (1.60 ml, 20.0 mmol). After stirring the reaction mixture at room temperature for 24 h, the mixture was poured into excess ethyl acetate. The resulting precipitate was dissolved in methanol and purified by selective precipitation from ethyl acetate. The yield of pure OS-mimBr was 0.98 g (74%). 1H-NMR (DMSO-d6): δ 0.090 (s, 6H, –SiCH3), 0.51 (m, 2H, –SiCH2–), 1.22 (m, 2H, –SiCH2CH2–), 1.42 (m, 1H, –SiCH2CH2CH2–), 1.50 (m, 1H, –SiCH2CH2CH2–), 2.51 (m, 2H, –OCOCH2–), 2.73 (m, 1H, –OCOCH–), 3.93 (s, 6H, –NCH3), 4.30–4.51 (m, 8H, –COOCH2CH2–), 7.83 (m, 4H, –NCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHN–), 9.41 (s, 2H, –NCH
CHN–), 9.41 (s, 2H, –NCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) ppm. 13C-NMR (DMSO-d6): δ −0.0749, 17.23, 20.27, 31.18, 35.34, 36.37, 60.23, 62.77, 64.43, 123.06, 124.04, 137.62, 171.76, 174.32 ppm. 29Si-NMR (DMSO-d6): δ 14.18, −108.48 ppm.
N–) ppm. 13C-NMR (DMSO-d6): δ −0.0749, 17.23, 20.27, 31.18, 35.34, 36.37, 60.23, 62.77, 64.43, 123.06, 124.04, 137.62, 171.76, 174.32 ppm. 29Si-NMR (DMSO-d6): δ 14.18, −108.48 ppm.
Imidazolium–TCNQ anion radical terminated OS-core dendrimer (OS-mimTCNQ)
OS-mimBr (0.300 g, 5.65 × 10−2 mmol) was dissolved in water. An aqueous solution of LiTCNQ (0.286 g, 1.37 mmol) was added to the dendrimer aqueous solution. The deep blue dendrimer precipitated out of solution. The precipitate was washed several times with water, until no residual LiTCNQ was detected with the FT-IR spectrum. The yield of OS-mimTCNQ was 0.29 g (70%). UV λmax (acetonitrile): 421, 744, 843 nm. FT-IR (KBr): υ = 2180, 2155, 1733, 1576, 1505, 1353, 1171, 1079 cm−1. Anal. calcd for C380N96O52H344Si16: C, 59.26; H, 4.75; N, 18.43%. Found: C, 59.50; H, 4.06; N, 20.18%.1-Methyl-3-n-propylimidazolium TCNQ anion radical (PmimTCNQ)
PmimTCNQ was obtained using PmimBr and LiTCNQ by following the similar method for OS-mimTCNQ. The yield of pure PmimTCNQ was 69%. UV λmax (acetonitrile): 421, 744, 843 nm. FT-IR (KBr): υ = 2191, 2175, 2152, 1587, 1509, 1362, 1182, and 1164 cm−1. Anal. calcd for C19N6H17: C, 69.28; H, 5.20; N, 25.51%. Found: C, 69.26; H, 5.22; N, 25.47%.Preparation of the calibration curve for the TCNQ anion radical
The calibration curve for the determination of the degree of introduction of the TCNQ anion radical in OS-mimTCNQ were prepared by using the absorbance of PmimTCNQ at 843 nm in acetonitrile solutions with concentrations of 5.95, 11.9, and 5.95 × 10−7 M. The calibration equation was Y = 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556C and correlation coefficient (R2) of 1.
556C and correlation coefficient (R2) of 1.
Preparation of cast films
The acetonitrile solution of neutral TCNQ was added to the acetonitrile solution of OS-mimTCNQ at 50 °C. The mixture solutions were casted on glass substrates and subsequent drying at 40 °C for 3 h.Results and discussion
Synthesis of imidazolium–TCNQ anion radical terminated OS-core dendrimer (OS-mimTCNQ)
The OS-core dendrimer with imidazolium–TCNQ anion radicals (OS-mimTCNQ) was synthesized from octakis(propenyl succinicanhydrido)OS (OS-SA) by the three step reactions (Scheme 1). First, the bromoethyl-terminated OS-core dendrimer (OS-Br) was synthesized via the ring-opening reaction and subsequent condensation reaction with 2-bromoethanol. OS-Br was identified based on 1H, 13C, and 29Si-NMR, and MALDI TOF-MS data (Fig. S1–4†). MALDI TOF-MS spectrum of OS-Br showed the presence of the peak at 4034.0 m/z, which agreed with the molecular mass of the fully functionalized dendrimer including potassium (4033.5 g mol−1). In the 1H-NMR spectrum for OS-Br, however, the introduction ratio of the ester groups was estimated to be 84% by comparing the integration ratio of the –CH2CH2Br with that of the –SiCH3. The next step involved the ionization of the terminal group of OS-Br using 1-methylimidazole to obtain the imidazolium–bromide terminated OS-core dendrimer (OS-mimBr), which was characterized based on 1H, 13C, and 29Si-NMR spectra (Fig. S5–7†). The 29Si-NMR spectrum of OS-mimBr showed two peaks corresponding to the silicate core unit and the dimethylsilyl unit (Fig. S7†), and no other peaks were detected, indicating no decomposition of the OS-core. The 1H-NMR spectrum of OS-mimBr showed that integration ratio of the protons on the N-methyl groups was 84% compared with integrations from the –SiCH3 (Fig. S5†). Finally, the imidazolium–TCNQ anion radical terminated OS-core dendrimer (OS-mimTCNQ) was prepared by anion exchange reaction of OS-mimBr with lithium TCNQ salt (LiTCNQ) in water. After a LiTCNQ aqueous solution was added to the aqueous dendrimer solution, precipitates were isolated by suction filtration and washed with water for several times. The FT-IR spectrum of the precipitates detected no band at 2208 cm−1 derived from the CN stretching band in LiTCNQ, suggesting no residual LiTCNQ was included in the product. The FT-IR spectrum of OS-mimTCNQ showed a new CN stretching band at 2180 cm−1, suggesting that the cation exchanging to the TCNQ anion radical (Fig. 2).3b,c,14 Other major bands were also observed at 1730 and 1073 cm−1 due to the ester and O–Si–O bonds, respectively. In addition, no desymmetrization and split of the stretching band of O–Si–O were detected, indicating that there was no decomposition of the OS core. | ||
| Fig. 2 (left) IR spectra of OS-mimTCNQ (a) and LiTCNQ (b), and (right) expanded spectra around the CN stretching band. | ||
The UV-vis spectrum of a acetonitrile solution of OS-mimTCNQ shows three characteristic peaks at 421, 744, and 843 nm, which are assigned to the TCNQ anion radical (Fig. 3).3c The degree of the introduction of the TCNQ anion radical in OS-mimTCNQ against the N-methyl groups was 95% estimated by the calibration curve method using the absorbance of 1-methyl-3-n-propylimidazolium TCNQ anion radical (PmimTCNQ) at 843 nm in the UV-vis spectra (Fig. S8†). The EDX pattern of OS-mimTCNQ includes no peaks due to Br (around 1.5 keV), indicating the absence of the unreacted terminal groups and the residual bromide anion species (Fig. S9†).
Preparation of the conductive casting films of OS-mimTCNQ with neutral TCNQ
An acetonitrile solution of the neutral TCNQ was added to an acetonitrile solution of OS-mimTCNQ at 50 °C. The feed molar ratios of the neutral TCNQ against the terminated imidazolium moiety in OS-mimTCNQ ([TCNQ]/[imidazolium]) were used the value of from 0.7 to 1.5 (OS-CTx; X = the feed molar ratios). The mixture solutions were casted on glass substrates and subsequently drying at 40 °C for 3 h. The film thickness of the casting films was around 40 μm as estimated by a micrometer caliper. The mixture solution for OS-CT1.0 was spin-coated on a polyvinyl chloride film and found that the film showed no cracking after bending the film (Fig. S10†).The conductivity of the casting films was evaluated with a four-probe technique as the average of the ten distinct points on the films. The conductivities of the casting films are summarized in Table 1. Although the casting film of OS-mimTCNQ showed low conductivity (<10−6 S cm−1), the casting films with the neutral TCNQ showed the relatively high electron conductivity. The casting film with the same feed molar ratio between the neutral TCNQ and the terminated imidazolium moiety (OS-CT1.0) showed the highest conductivity.
| Casting film | [TCNQ]/[imidazolium]a | Thicknessb, μm | Surface conductivity, ×105 Ω ◻−1 | Conductivity, S cm−1 |
|---|---|---|---|---|
| a The feed molar ratios of the neutral TCNQ against the terminated imidazolium moiety in OS-mimTCNQ.b Estimated by a micrometer caliper. | ||||
| OS-CT0.7 | 0.7 | 53 | 0.32 | 5.8 × 10−3 |
| OS-CT0.8 | 0.8 | 36 | 0.60 | 4.6 × 10−3 |
| OS-CT0.9 | 0.9 | 29 | 0.28 | 1.2 × 10−2 |
| OS-CT1.0 | 1.0 | 32 | 0.058 | 5.4 × 10−2 |
| OS-CT1.1 | 1.1 | 44 | 0.24 | 9.4 × 10−3 |
| OS-CT1.2 | 1.1 | 32 | 0.22 | 1.4 × 10−2 |
| OS-CT1.5 | 1.5 | 37 | 0.29 | 9.3 × 10−3 |
Stacking structures with the mixed-valence state in the casting films were confirmed by the absorption band around 2000 nm in the UV-vis-NIR absorption spectra (Fig. 4).5,13 In solid salts of TCNQ anion radical such as LiTCNQ, TCNQ dimer gives absorption bands near 360 nm and 640 nm, and that an intermolecular CT band is observed near 1100 nm.3c,12 The UV-vis NIR spectrum of the casting film of OS-mimTCNQ showed characteristic peaks at 380, 680, and 1100 nm assignable to the TCNQ dimer and the intermolecular charge transfer transition, respectively, while no absorption band of the mixed-valence state stacking was detected (Fig. 4a). In the UV-vis-NIR spectra of OS-mimTCNQ with the neutral TCNQ, the broad absorption peak in the region of 2000 nm was observed, indicating the existence of the mixed-valence state stacking in all the samples with the neutral TCNQ. The relative intensities of the absorption band of the TCNQ dimer decreased with increasing the X values from 0.8 to 1.0. This result indicates that the growth of the mixed valence state stacking from the charge transfer transition state against the TCNQ dimer proceeded by increasing the feed ratio of the neutral TCNQ. On the other hand, in the case of the excess feed ratio of the neutral TCNQ, the absorption bands of the TCNQ dimer around 680 nm again appeared, suggesting the mixed valence state stacking from the charge transfer transition state against the TCNQ dimer is inhibited. These results support that the casting film with the same feed molar ratio between the neutral TCNQ and the terminated imidazolium moiety (OS-CT1.0) showed the highest conductivity.
In the FT-IR spectrum of OS-CT1.0, the CN stretching band appeared as a broad peak in the region from 2120 to 2230 cm−1 (Fig. 5). In addition to the CN stretching bands of the neutral TCNQ at 2225 cm−1 and the OS-mimTCNQ at 2180 cm−1 and 2155 cm−1 due to B1u and B2u modes, respectively,14 the peak in the CN stretching region of OS-CT1.0 at 2193 cm−1 was assignable, which may be assignable to partially charge transfer TCNQ.15 In the case of other casting films of OS-mimTCNQ with the neutral TCNQ, similar CN stretching bands were observed and no obvious difference was observed in the FT-IR analysis (Fig. S11†).
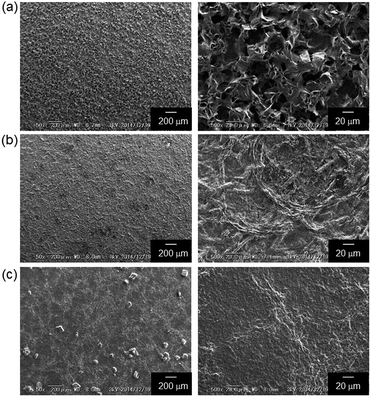
The surface morphology of the mixed-valence TCNQ constructed in the OS-core dendrimer films is observed by an SEM analysis (Fig. 6). The SEM image of the casting film of OS-CT1.0 showed a smooth surface. On the other hand, in the SEM image of OS-CT0.7, a rough surface was observed in comparison to that of OS-CT1.0. In the case of OS-CT1.5, some crystals were observed on its surface, suggesting the crystallization of the excess neutral TCNQ. These observations clearly show that the OS-core dendrimer can suppress the crystallization of the mixed-valence state TCNQ. However, the addition of the excess neutral TCNQ is unfavorable because of the formation of the insulating crystal.
To evaluate formation of nanostructures of the mixed-valence state staking in the casting films, GI-XD was carried out (Fig. 7). The XRD pattern of OS-minTCNQ shows a broad peak (Fig. S12†), suggesting an amorphous character. On the other hand, the GI-XD profiles of all samples showed characteristic peaks of 2θ at 10.8° (d-spacing = 8.2 Å) and 28.6° (d-spacing = 3.1 Å), corresponding to the plane spacing of TCNQ stacking and interplanar distance of TCNQ species, respectively.16 The diffraction peak of the octa-substituted caged silsesquioxanes shows a characteristic peak of 2θ at around 6° to 9° dependent on sizes of the substituents.17 In the present case, the diffraction peak at 6.6° showed an anisotropic pattern, indicating that the OS-core dendrimer arranged in the direction of the out-of-plane at 13.4 Å (Fig. 1). The diffraction peaks of 2θ higher than 10° show no obvious difference, suggesting no anisotropic orientation for the crystalline domains derived from neutral TCNQ and the terminated TCNQ anion radical in OS-minTCNQ.
 | ||
| Fig. 7 GI-XD profiles of OS-CT0.7 (a), OS-CT1.0 (b), and OS-CT1.5 (c); (A) in-plane and (B) out-of-plane. | ||
Conclusions
We synthesized the imidazolium–TCNQ anion radical terminated OS-core dendrimer (OS-mimTCNQ) and prepared the conductive films by casting the mixture of OS-mimTCNQ and the various amounts of the neutral TCNQ in acetonitrile on the substrates. The casting films with several tens of micron thickness showed the relatively high electron conductivity and the construction of the mixed-valence state stacking in these casting films was confirmed by UV-vis-NIR spectra. In particular, the casting film with the same feed molar ratio between the neutral TCNQ and the terminated imidazolium moiety (OS-CT1.0) showed the highest conductivity (5.4 × 10−2 S cm−1) in the present samples and good film formability. This study suggests that the OS-core dendrimers are ideal candidates as a scaffold for the construction of the continuous conductive pathway. Thus, the OS-core dendrimer solid materials have potential to be a platform for new series of nanomaterials.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No. 2401)” (JSPS KAKENHI Grant Number JP24102003) of The Ministry of Education, Culture, Sports, Science, and Technology, Japan. We thank Prof. Tsuyoshi Kawai of Nara Institute of Science and Technology for measuring MALDI-TOF-MS supported by Nanotechnology Platform.Notes and references
- J. B. Torrance, Acc. Chem. Res., 1979, 12, 79–86 CrossRef CAS.
- V. A. Starodub and T. N. Starodub, Russ. Chem. Rev., 2014, 83, 391–438 CrossRef.
- (a) A. Pearson, A. P. O'Mullane, S. K. Bhargava and V. Bansal, Langmuir, 2013, 29, 8–12 CrossRef CAS PubMed; (b) L. L. Martin, A. I. Siriwardana, J. Lu, X. Qu, C. Zhao and A. M. Bond, Aust. J. Chem., 2011, 64, 732–740 CAS; (c) S. Nešpůrek, I. Koropecký, M. Šorm, A. Graja and E. Sopa, Phys. Status Solidi B, 1987, 140, 545–558 CrossRef.
- S. Nešpůrek, L. Kalvoda, L. Machová, J. Pfleger and G. C. Stevens, Synth. Met., 1996, 82, 133–140 CrossRef.
- S. Terashita, K. Nakatsu, Y. Ozaki and S. Takagi, J. Phys. Chem., 1995, 99, 3618–3628 CrossRef CAS.
- K. Hayakawa, Y. Tsuji and K. Naka, Compos. Interfaces, 2013, 20, 1–14 CrossRef CAS.
- M. Kryszewski and J. Pęcherz, Polym. Adv. Technol., 1993, 5, 146–160 CrossRef.
- Y. Chujo and K. Tanaka, Bull. Chem. Soc. Jpn., 2015, 88, 633–643 CrossRef CAS.
- K. Naka, R. Shinke, M. Yamada, F. D. Belkada, Y. Aijo, Y. Irie, S. R. Shankar, K. S. Smaran, N. Matsumi, S. Tomita and S. Sakurai, Polym. J., 2014, 46, 42–51 CrossRef CAS.
- (a) Y. Irie and K. Naka, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 1437–1443 CrossRef CAS; (b) Y. Irie and K. Naka, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 628–633 CrossRef CAS.
- S. Pitula and A. V. Mudring, Chem.–Eur. J., 2010, 16, 3355–3365 CrossRef CAS PubMed.
- S. Skaria and S. Schricker, J. Macromol. Sci., Part A: Pure Appl. Chem., 2010, 47, 381–391 CrossRef CAS.
- L. R. Melby, R. J. Harder, W. R. Hertler, W. Mahler, R. E. Benson and W. E. Mochel, J. Am. Chem. Soc., 1962, 84, 3374–3387 CrossRef CAS.
- S. B. Khoo, J. F. Foley, C. Korzeniewski and S. Pons, J. Electroanal. Chem., 1987, 233, 223–236 CrossRef CAS.
- S. Nešpůrek, I. Koropecký, M. Šorm, A. Graja and E. Sopa, Phys. Status Solidi B, 1987, 140, 545–558 CrossRef.
- (a) R. Clérac, S. O'Kane, J. Cowen, X. Ouyang, R. Heintz, H. Zhao, M. J. Bazile Jr and K. R. Dunbar, Chem. Mater., 2003, 15, 1840–1850 CrossRef; (b) M. C. Grosse, P. B. Hitchcock, K. R. Seddon, T. Welton and S. C. Weston, Chem. Mater., 1994, 6, 1106–1108 CrossRef.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21636f |
| This journal is © The Royal Society of Chemistry 2016 |