Investigation of the fluorescence quenching behavior of PEI-doped silica nanoparticles and its applications†
Yali Qiao and
Xingwang Zheng*
Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi'an 710062, P. R. China. E-mail: zhengxw@snnu.edu.cn; Fax: +86-29-81530791; Tel: +86-29-81530791
First published on 27th September 2016
Abstract
Although the primary amine groups on PEI-based materials were widely used to bind fluorophores for optical sensing, the fluorescence quenching behavior of the tertiary amine groups on the same PEI macromolecules toward fluorophores was always ignored. To address these issues, in this work, we developed a new strategy that could not only avoid the limitation of the fluorescence quenching effect of the tertiary amino groups of PEI, but also keep the high reactivity of the primary amine groups. This new strategy was based on supramolecular integration of PEI into a silica matrix to form porous PEI-doped silica nanoparticles (PEI/silica nanoparticles). Our results showed that the primary amine groups of PEI inside porous PEI/silica nanoparticles possess great space freedom to maintain their high reactivity, but the tertiary amine groups of PEI were effectively masked based on the stronger supramolecular interaction between SiO− and the tertiary amino groups. Based on this finding, calcein/Co2+ fluorescence sensing systems were chosen as the model to demonstrate novel fluorescent properties of the porous PEI/silica nanoparticles. Our results showed that bright fluorescent porous calcein/PEI/silica nanoparticles (CPSNPs) could be easily prepared and further used to detect Co2+ based on the fluorescence quenching effect of Co2+ on calcein. A detection limit as low as 0.3 nM for Co2+ was achieved by these designed sensing nanomaterials.
Introduction
PEI, a cationic polymer, consists of a large number of amine functional groups and has been widely used for gene delivery,1–3 metal ions separation,4,5 blood purification,6 shampoo manufacturing,7 optical sensing8–14 and polymer dots preparation.15,16Of these applications, the optical sensing application was attractive because the amine groups of PEI (particularly primary amine groups) could well immobilize fluorescent receptor units on the PEI-based substrate by the excellent chemical activity of these amine groups.13 To date, many PEI-based fluorescence sensing platforms have been developed for detection of different analytes.17–23 Incorporation of small molecules with PEI polymer to prepare fluorescent probes was one of the general strategies for the fabrication of eminent PEI-based optical platforms.19,20 The evident advantage of these methods is their easy synthesis. Another typical strategy was grafting PEI onto the surface of the nanomaterials to serve as the linking reagent for immobilization of fluorescent receptor units.21–23 These methods have well utilized the high reactivity of the primary amine groups of PEI to immobilize fluorophores on PEI-based nanomaterials. However, it was noted that the residual amine groups of PEI (the secondary and tertiary amine groups) could quench the fluorescence of some fluorophores.24,25 In this case, construction of an optical sensing platform, based on PEI, also suffered from drawbacks in terms of practical applications.
This study aims to overcome the fluorescence quenching behavior of PEI. Herein we synthesized porous PEI/silica nanoparticles based on our previous work26 and systematically investigated the fluorescence quenching behavior of PEI and PEI/silica nanoparticles. Our results showed that the fluorescence quenching performance of PEI towards fluorophores mainly resulted from the protonation of the amine groups of PEI. The tertiary amine groups, which were much more easily protonated even in nearly neutral conditions, had a great impact on the fluorescence signals. Furthermore, it was found for the first time that, based on the supramolecular interaction between SiO− and the tertiary amine groups, the as-prepared porous PEI/silica nanoparticles could effectively avoid the fluorescence quenching performance of PEI in nearly neutral conditions. Finally, calcein, which was chosen as the model fluorescent receptor unit, had been self-assembled on the porous PEI/silica nanoparticles to construct a PEI-based fluorescence sensing platform for Co2+ detection. Our results showed that calcein was successfully self-assembled inside the prepared porous PEI/silica nanoparticles and formed a bright fluorescent PEI-based nanomaterial for Co2+ detection with a detection limit as low as 0.3 nM.
Experimental
Materials
Triton X-100, tetraethylorthosilicate (TEOS, 99.9%) and polyethyleneimine (PEI, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424 Da) were purchased from Sigma-Aldrich (United States); cyclohexane, acetone, ethanol and ammonium hydroxide (25–28 wt%) were obtained from Xian Chemical Reagent Factory; n-hexanol was purchased from Tianjin Chemical Reagent Factory; calcein and eosin Y were provided by Aladdin Reagent Co., Ltd. (Shanghai, China). A stock solution of calcein (1 × 10−3 M) was prepared by dissolving a suitable amount of calcein in ethanol. Milli-Q ultrapure water (18.2 MΩ) was used in all experiments. All other reagents and solvents were of analytical grade and were used as received without further purification.
424 Da) were purchased from Sigma-Aldrich (United States); cyclohexane, acetone, ethanol and ammonium hydroxide (25–28 wt%) were obtained from Xian Chemical Reagent Factory; n-hexanol was purchased from Tianjin Chemical Reagent Factory; calcein and eosin Y were provided by Aladdin Reagent Co., Ltd. (Shanghai, China). A stock solution of calcein (1 × 10−3 M) was prepared by dissolving a suitable amount of calcein in ethanol. Milli-Q ultrapure water (18.2 MΩ) was used in all experiments. All other reagents and solvents were of analytical grade and were used as received without further purification.
Instrumentation
A multi-position magnetic stirrer (IKA, Germany) was used for the preparation of the nanoparticles and a high-speed centrifuge (5804R, Eppendorf, Germany) was used for the collection of nanoparticles. Fluorescence spectra were measured with a fluorescence spectrophotometer (F-7000, Hitachi, Japan). UV-Vis absorption spectra were obtained by a UV-Vis spectrophotometer (U-3900, Hitachi). The transmission electron microscopy (TEM) images of the nanoparticles were measured with a Tecnai G2 F20 transmission electron microscope (FEI, America).Synthesis of porous PEI/Silica nanoparticles
PEI/silica nanoparticles were first prepared by a reversed microemulsion method.26,27 Then, the porous PEI/silica nanoparticles were fabricated by etching of PEI/silica nanoparticles with NaOH.Briefly, 250 μL of ultrapure water was added into the mixture of TX-100 (1.80 mL), cyclohexane (7.50 mL) and 1-hexanol (1.80 mL) to form a reverse microemulsion system. The mixture was stirred for about 30 min continuously. Then, 50 μL of PEI aqueous solution (0.01 g mL−1) was added into the mixture, followed by the addition of 90 μL of TEOS and 60 μL of NH4OH (25–28 wt%). After 30 hours, acetone was added into the mixture to release nanoparticles from the microemulsion. The resultant nanoparticles were collected by centrifuging and washing with ethanol and water several times.
Finally, the as-prepared PEI/silica nanoparticles were etched by NaOH (0.05 M) for about 15 min before reacting with eosin Y and calcein. Typically, PEI/silica nanoparticles were dispersed into pure water (4.5 mL), followed by the addition of 500 μL of 0.5 M NaOH into the nanoparticles suspension. The fabricated porous PEI/silica nanoparticles were collected by centrifuging (8000 rpm) and washing with water several times.
Fluorescence quenching efficiency measurement
For all florescence spectra, measurement was conducted on a Hitachi F-7000 fluorescence spectrophotometer. Typically, 20 μL of PEI solution (0.01 g mL−1) was added into 1 mL of eosin Y solution, then the fluorescence spectra of eosin Y solution and PEI-eosin Y mixture were recorded. Furthermore, the fluorescence quenching efficiency of PEI/silica nanoparticles to eosin Y was measured by the addition of 20 μL of nanoparticles' suspension (dispersed into 1 mL pure water) into 1 mL of eosin Y solution. In all cases, the excitation wavelength was set at 512 nm and the slit widths of the excitation and emission radiation were both set at 5 nm.Self-assembly of calcein on the porous PEI/Silica nanoparticles
Briefly, the as-prepared porous PEI/silica nanoparticles (0.7 mg) were dispersed into 1 mL pH 8.0 sodium phosphate buffer solution (PB, 10 mM), and then 200 μL of calcein solution (1 × 10−4 M) was added. After mixing well with shaking, the mixture was placed in the darkroom at room temperature for 40 min. The resultant porous calcein/PEI/silica nanoparticles (CPSNPs) were collected by centrifuging and washing with pure water several times to remove free-state calcein.Results and discussion
Investigation of fluorescence quenching behavior of PEI solution toward eosin Y
Eosin Y was chosen as the model anionic dye to investigate the fluorescence quenching behavior of PEI for fluorophores. The fluorescence quenching efficiency of PEI solution to eosin Y in different pH solutions was measured, as shown in Fig. 1; when the pH values decreased from 10.0 to 4.0, the fluorescence quenching efficiency of PEI to eosin Y increased gradually. Once the pH was down to 4.0, the fluorescence quenching efficiency did not change anymore. Although the fluorescence quenching efficiency of PEI to eosin Y was higher at lower pH, fluorescence intensity of eosin Y was very weak at pH 4.0 and 2.0 solutions (as shown in the inset of Fig. 1). These results suggested that the fluorescence quenching efficiency of PEI to eosin Y could be regulated by changing the pH value of the buffer solutions. According to previous literature,25 the fluorescence of eosin Y may be quenched by PEI through a photoinduced electron transfer (PET) process. The pKa values of the three types of amine groups of PEI are 4.5 for primary, 6.7 for secondary and 11.6 for tertiary, which means that the ratio of protonated amine groups can be modulated by changing the pH (as shown in Scheme 1).28,29 The positive charge of PEI increased as the pH decreased; subsequently, PEI strongly interacted with eosin Y in the solutions of lower pH and reduced the fluorescence of eosin Y.Characterization of the synthesized porous PEI/Silica nanoparticles
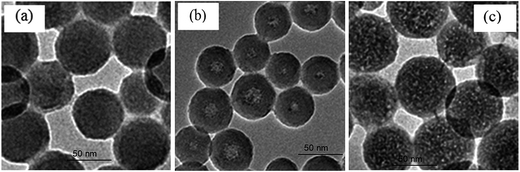
To improve the optical properties of PEI-based nanomaterials and broadening their application in bioanalysis it is of great importance to reduce the quenching behavior of PEI toward fluorophores in nearly neutral conditions. The strategy of decreasing PEI quenching performance, based on our previous work, was by doping of PEI into silica matrix using a reversed microemulsion method.26In order to confirm that PEI had successfully doped into the silica matrix, two types of PEI/silica nanoparticles were synthesized by the addition of different concentrations of PEI during the preparation process. The architectures of the as-prepared PEI/silica nanoparticles were studied in detail. TEM images indicated that the as-prepared PEI/silica nanoparticles (0.01 g mL−1 of PEI was used) were well dispersed, with an average size of about 65 ± 3 nm (Fig. 2a). However, when 0.05 g mL−1 of PEI was used, the resultant nanoparticles exhibited PEI core-silica shell structures and the sizes decreased slightly (Fig. 2b). The TEM images of the two kinds of nanoparticles better illustrated that PEI had successfully doped into the silica matrix. The PEI/silica nanoparticles synthesized by addition of 0.01 g mL−1 of PEI into the reversed microemulsion system were chosen for the later work.
In addition, the as-prepared PEI/silica (synthesized by the addition of 0.01 g mL−1 of PEI) nanoparticles were treated with an NaOH etching before investigation of their fluorescence quenching behavior, with the purpose of generating pores on the silica matrix to allow eosin Y to enter into the silica matrix and react with the exposed amine groups of PEI.26 As shown in Fig. 2c, after etching by NaOH, the PEI/silica nanoparticles had a porous structure.
Investigation of fluorescence quenching behavior of the porous PEI/Silica nanoparticles
The surface property of the porous PEI/silica nanoparticles in the different pH solutions was investigated by conducting ζ potential measurement. In pH 10.2 and 8.0 solutions, the ζ potentials of the porous PEI/silica nanoparticles were approximately equal with values of about 12.14 mV and 14.11 mV, respectively. This result indicated that the tertiary amine groups of PEI in the porous PEI/silica nanoparticles cannot be protonated. The possible reason was that the tertiary amine groups of PEI had reacted with the silica matrix based on the stronger supramolecular interaction between SiO− and the tertiary amine groups. However, when the pH was 6.7 and 4.5, the ζ potentials of the porous PEI/silica nanoparticles were 30.11 mV and 49.23 mV, respectively. The increase of the ζ potential is attributed to the protonation of the primary and secondary amine groups of PEI inside the PEI/silica nanoparticles. All of the measured results suggested that the primary amine groups of PEI in the PEI/silica nanoparticles retained their reactivity, but the tertiary amine groups were masked.Based on the results mentioned above, we explored whether the fluorescence quenching effect of PEI had been avoided by the as-prepared porous PEI/silica nanoparticles. As shown in Fig. 3, in pH 8.0 buffer solution, the fluorescence intensity of eosin Y was quenched about 34.56% by PEI solution (black columns). However, when the same amount of PEI was doped into silica nanoparticles, the fluorescence quenching efficiency was only about 6.56% (blue columns). When pH decreased stepwise from 8.0 to 2.0, the quenching efficiency of PEI/silica nanoparticles increased gradually and finally almost reached to that of PEI. In addition, the red columns in Fig. 3 showed that, after treating porous PEI/silica nanoparticles with HF to destroy the silica matrix, the quenching efficiency recovered. These results suggested that the PEI/silica nanoparticles well overcome the quenching effect of PEI on eosin Y under nearly neutral conditions. Because the tertiary amine groups of PEI had reacted with SiO− groups, the interaction between PEI/silica nanoparticles and eosin Y was too weak to quench the fluorescence of eosin Y under nearly neutral conditions.
 | ||
| Fig. 3 The fluorescence quenching efficiency of PEI solution (black), PEI/silica nanoparticles treated by HF (red) and PEI/silica nanoparticles (blue) toward eosin Y in different pH buffer solutions. | ||
A plausible mechanism for the decrease of the quenching efficiency of PEI towards eosin Y
As shown in Scheme 2, an experimentally simple strategy for the masking of PEI quenching behavior towards eosin Y in nearly neutral medium has been described based on the above results. In nearly neutral medium, the tertiary amine groups of PEI were protonated and then interacted with anionic eosin Y. Subsequently, the fluorescence of eosin Y was quenched by PEI through a photoinduced electron transfer (PET) process.25 However, compared to PEI in solution, when PEI was doped into silica nanoparticles, most of the tertiary amine groups of PEI firstly reacted with SiO− groups and were encapsulated into the silica matrix. Thus, in nearly neutral medium the tertiary amine groups of PEI did not interact with eosin Y to induce the fluorescence quenching. | ||
| Scheme 2 Illustration of the plausible mechanism for the decrease of the quenching efficiency of PEI to eosin Y. | ||
Self-assembly of calcein inside porous PEI/Silica nanoparticles
Calcein was chosen as the model fluorescent receptor unit in this study. On one hand, compared with eosin Y, a calcein molecule contains four carboxyl groups (as shown in Fig. S1 in the ESI†). The stronger interactions between calcein and PEI make it more stable for the immobilization of calcein inside the porous PEI/silica nanoparticles; on the other hand, calcein, a tetradentate ligand with four carboxyl groups, supplies abundant binding sites for ions.30PEI as a cationic polymer possesses several amine groups; when a suitable amount of calcein was added into the porous PEI/silica nanoparticles suspension, due to the stronger hydrogen bonding and electrostatic interactions between the carboxyl groups on calcein and the amine groups (especially primary amine groups) of PEI, calcein could be self-assembled inside the porous PEI/silica nanoparticles. ζ potential measurement was conducted to validate that calcein had been successfully assembled inside the porous PEI/silica nanoparticles. In pH 7.0 PB solution, ζ potential of the porous PEI/silica nanoparticles was 27.04 mV. However, after interaction of calcein with the porous PEI/silica nanoparticles for about 40 min, the white PEI/silica nanoparticles turned yellow and the ζ potential decreased to 7.04 mV under the same conditions. These results indicated that calcein was successfully assembled inside the porous PEI/silica nanoparticles and the yellow-pink calcein/PEI/silica nanoparticles (CPSNPs) were fabricated (as shown in Fig. S3 in the ESI†). In addition, the morphology of the CPSNPs was studied. After self-assembly of calcein inside of the porous PEI/silica nanoparticles, the fabricated nanoparticles still had good dispersity and the sizes were similar to the PEI/silica nanoparticles (as shown in Fig. S2†).
Fluorescence quantum yield of CPSNPs, PEI/Calcein mixture (PEI/Calcein), and free-state calcein
To determine whether the PEI and PEI/silica nanoparticles had influences on the florescence of calcein, the quantum yields of CPSNPs, PEI/calcein and free-state calcein were determined with quinine sulfate (ΦF = 0.52 in 0.05 M H2SO4) as the standard. As shown in Table S1,† in pH 8.0 PB solution, the quantum yield of calcein was 32.4%, after self-assembly of calcein inside the PEI/silica nanoparticles; the quantum yield of CPSNPs was 25.1% under the same conditions. However, when calcein was mixed with PEI solution for 40 min, the quantum yield of PEI/calcein solution evidently decreased and was only 9.7%. These results implied that PEI in solution had a great impact on the fluorescence of calcein. However, the influence of PEI could be weakened effectively in nearly neutral conditions by doping of PEI into the silica matrix.Fluorescence response of CPSNPs to Co2+
Under the optimal conditions, the fluorescence response of CPSNPs toward different concentrations of Co2+ was investigated. The fluorescence spectra of CPSNPs (λex = 495 nm, λem = 518 nm) versus different Co2+ concentrations are shown in Fig. 4; as the concentration of Co2+ increased from 1 × 10−9 M to 9 × 10−7 M, the fluorescence intensity of CPSNPs decreased gradually. The plot of F0/F versus Co2+ in the inset of Fig. 4 shows that a good linear correlation was obtained over the Co2+ concentration range from 1 × 10−9 M to 9 × 10−9 M, and KSV for Co2+ was determined to be 2.7 × 107 L mol−1. The related regression equation was y = 2.7 × 107x + 1.0084 (R = 0.9980).Furthermore, the possible mechanism of Co2+ quenching of the fluorescence of CPSNPs was investigated. Fig. 5 showed that, compared with the Co2+ solution, a broad absorption band ranging from 425 to 700 nm was observed after addition of the Co2+ solution into PEI. The absorption band of the Co2+ complex overlapped the emission band of calcein, thus, the fluorescence of CPSNPs was decreased in the presence of Co2+ through a fluorescence resonance energy transfer (FRET) process.22,31
 | ||
| Fig. 5 UV-Vis spectra of: (red) 50 μM of Co2+; (black) 1 mg mL−1 of PEI; (blue) PEI after chelation with Co2+. | ||
Selectivity
The specificity of this Co2+ detection method was measured by investigation of the fluorescence changes in the presence of other 10-fold co-existing metal ions (Zn2+, Ca2+, Ag+, K+, Pb2+, Mg2+, Cu2+, Cd2+, Al3+, Ni2+, Hg2+ and Ba2+). As shown in Fig. 6, among these metal ions, Hg2+ and Ag+ had influences on the Co2+ detection. In order to avoid the interference of Ag+ and Hg2+, 10 mM NaCl was added into the buffer solution. In the presence of Cl−, due to the formation of precipitates (AgCl) and complexation of Hg2+ by Cl−, the concentration of Ag+ and Hg2+ decreased. As shown in Fig. S4,† the influences of Hg2+ and Ag+ were overcome successfully by the addition of 10 mM NaCl.22,32,33Detection of Co2+ in tap water samples
To evaluate the practicality of our proposed Co2+ detection method, a standard addition method was applied to detect Co2+ in tap water samples. The tap water samples were obtained from our lab and were treated with a membrane before conducting Co2+ measurement. Good recovery was obtained by the proposed method, as shown in Table 1, with recovery of 95%, 95.3% and 100.8% for three determinations.| Sample | Added (10−8 M) | Detected (10−8 M) | Recovery (%) |
|---|---|---|---|
| Tap water | 3.00 | 2.85 | 95.0 |
| 5.00 | 5.04 | 100.8 | |
| 7.00 | 6.67 | 95.3 |
Conclusion
In this study, the fluorescence quenching behavior of the tertiary amine groups of PEI in solution and in a silica nanomatrix towards fluorophores was studied for the first time. The SiO− groups inside the silica nanomatrix were found to selectively mask the tertiary amine groups of PEI therefore, decreasing its fluorescence quenching effect. This finding may open up a new way for designing new functional PEI-based nanomaterials for improving their optical sensing performance. Based on this proposed selective chemical masking mechanism, this strategy may be extended to design other new functional silica-based nanomaterials using different amino-based polymers (such as dendrimers).Acknowledgements
This work was financially supported by the projects from National Natural Science Foundation of China (No. 21375085) and the Fundamental Research Funds for the Central Universities (No. 2016CBZ003).Notes and references
- C. Y. Gao, H. Zhang, M. Wu, Y. Liu, Y. P. Wu, X. L. Yang and X. Z. Feng, Polym. Chem., 2012, 3, 1168–1173 RSC.
- Z. Z. Chen, L. F. Zhang, Y. L. He and Y. F. Li, ACS Appl. Mater. Interfaces, 2014, 6, 14196–14206 CAS.
- H. Yamada, B. Loretz and C. M. Lehr, Biomacromolecules, 2014, 15, 1753–1761 CrossRef CAS PubMed.
- C. Bertagnolli, A. Grishin, A. Vincent and E. Guibal, Ind. Eng. Chem. Res., 2016, 55, 2461–2470 CrossRef CAS.
- H. Bessbousse, T. Rhlalou, J.-F. Verchère and L. Lebrun, J. Membr. Sci., 2008, 325, 997–1006 CrossRef CAS.
- N. J. Kaleekkal, A. Thanigaivelan, M. Durga, R. Girish, D. Rana, P. Soundararajan and D. Mohan, Ind. Eng. Chem. Res., 2015, 54, 7899–7913 CrossRef CAS.
- L. F. W. Vleugels, J. Pollet and R. Tuinier, J. Phys. Chem. B, 2015, 119, 6338–6347 CrossRef CAS PubMed.
- K. Kim, J. W. Lee and K. S. Shin, ACS Appl. Mater. Interfaces, 2012, 4, 5498–5504 CAS.
- J. R. Zhang, Z. L. Wang, F. Qu, H. Q. Luo and N. B. Li, J. Agric. Food Chem., 2014, 62, 6592–6599 CrossRef CAS PubMed.
- Y. Zhang, J. M. Liu and X. P. Yan, Anal. Chem., 2013, 85, 228–234 CrossRef CAS PubMed.
- F. Qu, N. B. Li and H. Q. Luo, Langmuir, 2013, 29, 1199–1205 CrossRef CAS PubMed.
- J. M. Janjic, M. Srinivas, D. K. K. Kadayakkara and E. Ahrens, J. Am. Chem. Soc., 2008, 130, 2832–2841 CrossRef CAS PubMed.
- Y. Pan, Y. P. Shi, Z. H. Chen, J. Y. Chen, M. F. Hou, Z. P. Chen, C. W. Li and C. Q. Yi, ACS Appl. Mater. Interfaces, 2016, 8, 9472–9482 CAS.
- A. Masotti, P. Vicennati, F. Boschi, L. Calderan, A. Sbarbati and G. Ortaggi, Bioconjugate Chem., 2008, 19, 983–987 CrossRef CAS PubMed.
- Y. Ling, F. Qu, Q. Zhou, T. Li, Z. F. Gao, J. L. Lei, N. B. Li and H. Q. Luo, Anal. Chem., 2015, 87, 8679–8686 CrossRef CAS PubMed.
- Y. Q. Dong, R. X. Wang, G. L. Li, C. Q. Chen, Y. W. Chi and G. N. Chen, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- T. Xia, M. Kovochich, M. Liong, H. Meng, S. Kabehie and S. George, ACS Nano, 2009, 3, 3273–3286 CrossRef CAS PubMed.
- Y. P. Chen and X. D. Li, Biomacromolecules, 2011, 12, 4367–4372 CrossRef CAS PubMed.
- M. Y. Liu, J. Z. Ji, X. Y. Zhang, X. Q. Zhang, B. Yang, F. J. Deng, Z. Li, K. Wang, Y. Yang and Y. Wei, J. Mater. Chem. B, 2015, 3, 3476–3482 RSC.
- D. Ding, R. T. K. Kwork, Y. Y. Yuan, G. X. Feng, B. Z. Tang and B. Liu, Mater. Horiz., 2015, 2, 100–105 RSC.
- J. M. Rosenholm, A. Meinander, E. Peuhu, R. Niemi, J. E. Eriksson, C. Sahlgre and M. Lindén, ACS Nano, 2009, 3, 197–206 CrossRef CAS PubMed.
- C. H. Zong, K. Ai, G. Zhang, H. W. Li and L. H. Lu, Anal. Chem., 2011, 83, 3126–3132 CrossRef CAS PubMed.
- P. Yang, Y. Zhao, Y. Lu, Q. Z. Xu, X. W. Xu, L. Dong and S. H. Yu, ACS Nano, 2011, 5, 2147–2154 CrossRef CAS PubMed.
- M. A. Winnik and S. M. Bystryak, Macromolecules, 1999, 32, 624–632 CrossRef CAS.
- T. Wen, N. B. Li and H. Q. Luo, Anal. Chem., 2013, 85, 10863–10868 CrossRef CAS PubMed.
- Y. L. Qiao and X. W. Zheng, Analyst, 2015, 140, 8186–8193 RSC.
- R. P. Bagwe, C. Y. Yang, L. R. Hilliard and W. H. Tan, Langmuir, 2004, 20, 8336–8342 CrossRef CAS PubMed.
- K. D. Demadis, K. Paspalaki and J. Theodorou, Ind. Eng. Chem. Res., 2011, 50, 5873–5876 CrossRef CAS.
- M. Amara and H. Kerdjoudj, Talanta, 2003, 60, 991–1001 CrossRef CAS PubMed.
- Z. Y. Meng, C. D. Jiang, X. L. Li and J. Zhai, ACS Appl. Mater. Interfaces, 2014, 6, 3794–3798 CAS.
- A. W. Varnes, R. B. Dodson and E. L. Wehry, J. Am. Chem. Soc., 1972, 94, 946–950 CrossRef CAS PubMed.
- J. L. Yao, K. Zhang, H. J. Zhu, F. Ma, M. T. Sun, H. Yu, J. Sun and S. H. Wang, Anal. Chem., 2013, 85, 6461–6468 CrossRef CAS PubMed.
- S. Serrano, D. Vlassopoulos, B. Bessinger and P. A. O'Day, Environ. Sci. Technol., 2012, 46, 6767–6775 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21543b |
| This journal is © The Royal Society of Chemistry 2016 |