Stereoselective glycoconjugation of steroids with selenocarbohydrates†
Ricardo F. Affeldtac,
Francisco P. Santosa,
Rafael S. da Silvab,
Oscar E. D. Rodriguesb,
Ludger A. Wessjohannc and
Diogo S. Lüdtke*a
aInstitute of Chemistry, Universidade Federal do Rio Grande do Sul, Av. Bento Gonçalves 9500, 91501-970, Porto Alegre, RS, Brazil. E-mail: dsludtke@iq.ufrgs.br
bUniversidade Federal de Santa Maria, Santa Maria, RS, Brazil
cLeibniz Institute of Plant Biochemistry, Halle (Saale), Germany
First published on 26th September 2016
Abstract
A methodology that brings together sugar and steroid scaffolds linked by a selenium atom is discussed in this work. A series of 6β and 3α glycoconjugated steroids were achieved by stereoselective nucleophilic substitution of cholesterol, pregnenolone, stigmasterol and sitosterol with different seleno-pyranosides and furanosides.
Introduction
Steroidal glycosides including phytosterols are important biomolecules in which one or more carbohydrate units are attached to a steroid skeleton.1 For example, digitoxin and digoxin, extracted from Digitalis purpurea and lanata, are cardiac steroidal glycosides applied for heart failure by inhibition of Na+ K+ ATPase.2 They have also shown anti-cancer activity against breast and lung cancer cells.3 The carbohydrate moiety is known for modulating the binding on ATPase, therefore influencing its efficiency and modifications on these groups has been described to increase its biological activity substantially.4–6 The metabolism of cardiac glycosides is dependent of the lability of the glycosyl linkage with the steroidal molecule and the carbohydrate moiety being responsible for the appropriate solubility of the molecule in the biological media and to play a role in the binding activity. In addition, recent publications7 revealed that spirostan saponins (triterpene steroidal glycosides) feature potent cytotoxic activity against human leukemia cell lines HL-60, and antifungal, anti-inflammatory and antiviral activities.8 The substitution of oxygen by sulfur or selenium in conjugate linkages of biomolecules has been used frequently to reduce or slow down enzymatic breakage of this bond, and to enable bioconjugations not possible otherwise.9Much of the synthetic efforts towards steroid glycosides are based on modification of the sugar moiety and its introduction on the cholesterol framework by selective glycosylation reactions. Few strategies that employ modification on the traditional sugar–steroid linkage are reported for biological evaluation. Some interesting examples involve conjugation of the glycosyl and steroid units by Cu-catalyzed 1,3-dipolar cycloaddition between a sugar azide and an alkynyl steroid.10 However, the resulting triazole linked products did not maintain the same cytotoxic profile of the parent molecules. In addition, the Ugi multicomponent reaction was also used to construct a pseudo-peptide linkage between the sugar and spirostane moieties.11 This strategy afforded a library of glycoconjugated derivatives with unique architectures and wide substrate scope.
On the other hand, organoselenium compounds have received special attention by the scientific community, especially due the biological activity of sulfur-related molecules such as selenoenzymes.12 In this context, our groups have been involved with the introduction of organoselenium moieties into the structure of carbohydrates and their derivatives, affording new classes of selenium-containing xylo-furanosides,13 galacto-pyranosides,14 uridine derivatives,15 pseudodisaccharides and neoglycoconjugates.16 Some of our arylseleno-xylo-furanosides have shown particular bioactivity protecting against Cd and Mn poisoning17 and promising therapeutic agents for Alzheimer's disease as well.18
The introduction of organoselenium groups at the steroid scaffold has been scarcely reported in the literature and the few examples reported were used either as synthetic intermediates for the synthesis of hydroxylated steroids,19 as a radioactive 75Se imaging agent for the study of the distribution of cholesterol in different animal tissues,20 and more recently as liquid crystals.21 A relevant example describes an efficient synthesis of selenosteroids by the reaction of aromatic diselenides with cholesterol epoxides.22,23
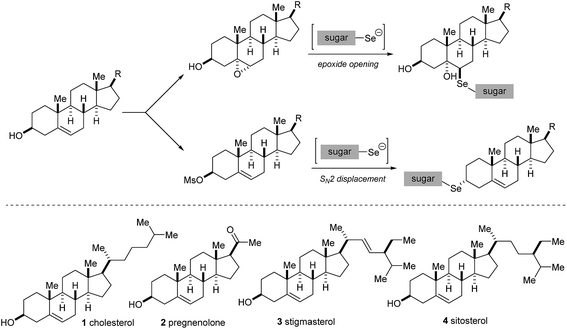
In this work we report the synthesis of novel selenium-linked steroidal glycoconjugates, starting from protected-D-carbohydrate diselenide derivatives and different modified human steroids (cholesterol and pregnenolone) as well as plant steroids (stigmasterol and sitosterol). A range of selenium-containing products was obtained either by the ring-opening of epoxides or SN2 displacement with selenium–sugar nucleophiles (Scheme 1).
Results and discussion
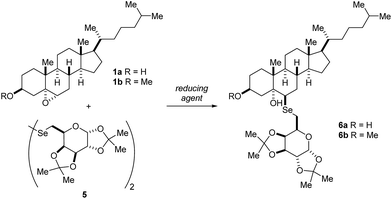
We began our investigation using the diselenide 5, derived from D-galactose and cholesterol epoxides 1a and 1b, which were prepared by a diastereoselective epoxidation of the double bond with m-CPBA.24 The reactive organoselenium species was generated in situ by the reductive cleavage of the diselenide bond, to generate 2 equivalents of the corresponding organoselenium nucleophile (Table 1). By using the traditional NaBH4 to promote reductive cleavage of the Se–Se bond in ethanol or THF/ethanol as solvent, the product was observed in less than 5% yield (entry 1). Since re-oxidation of the selenolate to diselenide was observed, successive additions of the reducing agent and longer reaction time led to increased yield of the desired steroidal selenoglycoconjugate (entry 3).25 Performing the reaction in the absence of THF afforded the product less effectively (entry 4). Is worth pointing out that 1.8 equivalents of the epoxide were needed to afford reasonable yields of product 6a. Changing the solvent to DMF, a more polar and aprotic solvent, was also examined (entries 5–8). Gratifyingly, under these conditions the desired product was obtained in 60% yield with a single addition of NaBH4 and using equimolar amounts of the epoxide 1a and the selenium nucleophile (entry 6). Further additions of the reducing agent did not result in any improvement of product formation (entry 7).| # | Epoxide (equiv.) | Reducing agent (equiv. × additions) | Solvent | Temp (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Isolated yield.b Reaction performed using 0.1 mmol of the sugar diselenide, 0.2 mmol of the epoxide, 0.4 mmol of NaBH4, in 2 mL of dry DMF.c With ZnCl2 (1.8 equiv.). | ||||||
| 1 | 1a (1.8) | NaBH4 (4.0 × 1) | THF/EtOH | 66 | 48 | <5 |
| 2 | 1a (1.8) | NaBH4 (2.5 × 3) | THF/EtOH | 66 | 24 | 37 |
| 3 | 1a (1.8) | NaBH4 (2.5 × 3) | THF/EtOH | 66 | 48 | 65 |
| 4 | 1a (1.8) | NaBH4 (2.5 × 3) | EtOH | 76 | 48 | 50 |
| 5 | 1a (1.8) | NaBH4 (4.0 × 1) | DMF | 80 | 48 | 60 |
| 6b | 1a (1.0) | NaBH4 (4.0 × 1) | DMF | 80 | 48 | 60 |
| 7 | 1a (1.0) | NaBH4 (4.0 × 3) | DMF | 80 | 48 | 53 |
| 8c | 1a (1.0) | NaBH4 (4.0 × 3) | DMF | 80 | 48 | <5 |
| 9 | 1a (1.8) | LiEt3BH (2.0 × 1) | THF | 66 | 48 | 50 |
| 10 | 1a (1.8) | LiAlH4 (2.0 × 1) | THF | 66 | 48 | <5 |
| 11 | 1b (1.0) | NaBH4 (4.0 × 1) | DMF | 80 | 48 | 32 |
| 12 | 1b (1.0) | NaBH4 (4.0 × 1) | DMF | 120 | 48 | 43 |
| 13c | 1b (1.0) | NaBH4 (4.0 × 1) | DMF | 80 | 48 | <5 |
| 14 | 1b (1.0) | NaBH4 (4.0 × 1) | THF/EtOH | 66 | 48 | n.r. |
| 15 | 1b (1.0) | NaBH4 (2.5 × 3) | THF/EtOH | 66 | 48 | 46 |
We tried to increase the reaction yield by using ZnCl2 as a Lewis acidic additive (entry 8), however it resulted in almost no reaction at all, presumably due to stabilization of the selenium nucleophile. Additional attempts changing the reducing agent to LiEt3BH and LiAlH4 only resulted in diminished yields of the product (entries 9 and 10). Thus, the following reaction conditions were defined as optimal and employed for further screenings: 1.0 equiv. of cholesterol epoxide 1a, 0.5 equiv. of the sugar diselenide 5 (after cleavage, 1.0 equiv. of the corresponding selenolate anion is generated), single addition of NaBH4 as the reducing agent, dry DMF for 48 h at 80 °C (entry 6). When the reaction was performed using cholesterol epoxide derivative 1b, a slightly better yield was achieved using the THF/ethanol mixture as solvent instead of DMF (compare entries 12 and 15). However, three successive additions of NaBH4 were required in order to achieve a reasonable yield of the product 6b.
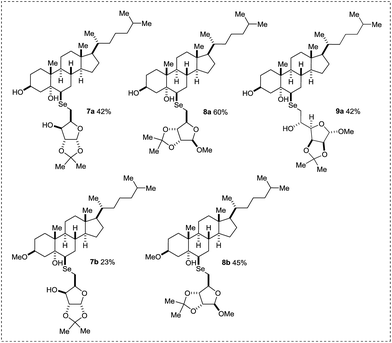
The scope of our reaction was further expanded to additional substrates, and the structures of the products are depicted in Fig. 1. We performed the reaction using the optimized conditions depicted in Table 1, entry 6, to three additional sugar diselenides, which were derived from D-xylose (Fig. 1, compounds 7a and 7b), D-ribose (Fig. 1, compounds 8a and 8b), and D-mannose (Fig. 1, compound 9a). All steroidal selenoglycoconjugates were obtained as single isomers through a regioselective nucleophilic epoxide opening (Fig. 1).
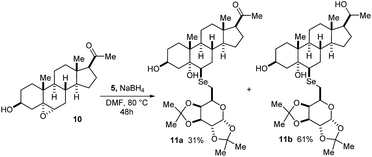
Interesting to note is that when pregnenolone epoxide 10 was used as the substrate for the reaction with D-galactose diselenide, a mixture of 6-β-selenoglycoconjugate ketone 11a and the corresponding alcohol 11b was obtained in 31% and 61% yield, respectively (Scheme 2). The formation of product 11b is explained as the result of the reduction of C20 ketone carbonyl of 11a by the excess of NaBH4.14 Attempts to lower the amount of the reducing agent in order to maintain the ketone unreacted resulted in slow reaction and unreacted 10 was recovered.
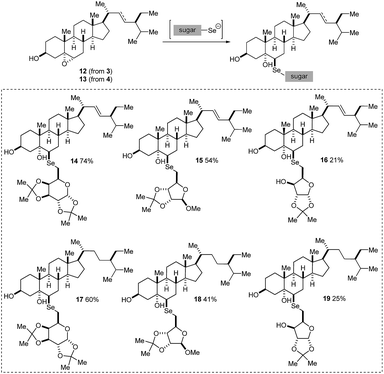
Further application of our methodology for glycoconjugation of selenocarbohydrates with steroid derivatives was carried out using plant steroids. Stigmasterol 3 and sitosterol 4 are abundant phytosteroids found in several plant extracts and are widely used as cholesterol mimics in food additives.26 In addition, several glycoside forms of these steroids have been reported to possess relevant bioactivities.27 Diastereoselective epoxidation with m-CPBA of both compounds has been performed to deliver the desired corresponding products 12 and 13 in good yields.24d In the case of stigmasterol, traces of a double epoxidation product was also isolated (concomitant epoxidation of the side chain double bond). Therefore, epoxide 12 was reacted with sugar diselenide 5, under our previously optimized conditions and the corresponding product 14 was isolated in 74% yield (Scheme 3). We have expanded the scope of the reaction by synthesizing six derivatives from stigmasterol and sitosterol through stereoselective epoxide opening with different sugar diselenides. The desired products were obtained in good yields, except when the xylose-derived diselenide was used, which resulted in diminished yields of products 16 and 19.
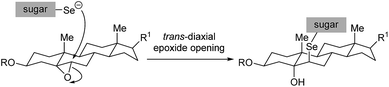
In all cases studied, the mechanism of the reaction proceeded through a regio- and stereoselective ring-opening of the epoxide with the in situ generated selenium nucleophile in a trans-diaxial fashion therefore delivering the product with the selenium atom attached to the 6β position of the steroid framework (Scheme 4). This assignment has been confirmed by NMR studies (see next section).
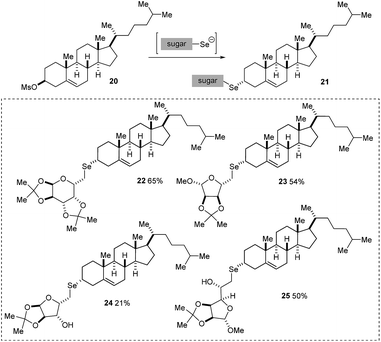
A similar strategy has proved useful for the synthesis of 3-cholesterol derivatives by reacting sugar diselenides with cholesteryl-3-mesylate 20. In these reactions, we have been able to prepare four new seleno-glycoconjugates in which the sugar moiety is now attached to the α-face of the molecule (Scheme 5).
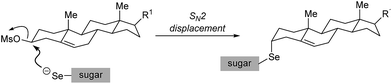
For the 3α-derivatives, the reaction is believed to proceed through a direct SN2 displacement, with stereoinversion, delivering the product with the organoselenium moiety at the 3β position of the steroid (Scheme 6). Detailed NMR experiments support this assignment (see next section).
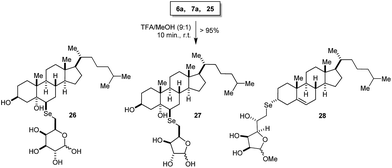
Finally, it is important to highlight that these compounds are readily deprotected in the presence of trifluoroacetic acid and methanol leading to free hydroxyl derivatives (Scheme 7) evidenced by disappearance of the isopropylidene signals on 1H NMR spectra (0.5–1.5 ppm, see ESI†).
NMR studies
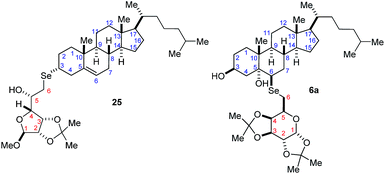
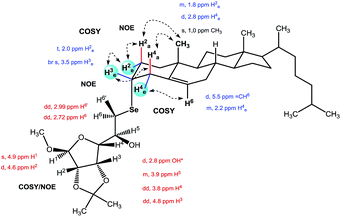
Attempts to achieve crystals suitable for X-ray diffractometry of the Se-glycoconjugated products were unsuccessful, leading to oil or semisolid physical states. In order to properly attribute the stereochemistry of the new center formed by the nucleophilic displacement of 3β-cholesteryl mesylate or ring opening of the 5,6α-cholesterol epoxide, two-dimensional NMR experiments were performed (COSY, HSQC, and NOESY) in a 400 MHz magnet. The spectral data for selected compounds 6a and 25 will be discussed here (complete NMR data achieved are included in the ESI† section), with the atoms labeled according to the official rules for each steroid and carbohydrate moieties (Fig. 2).For compound 25 which bears 62 hydrogen and 37 carbon atoms, some 1H-NMR signals are easily attributed by comparison with the starting materials X and Y spectra, such as the olefin H6 (d, 5.4 ppm, 3JH6,H7 = 4.6 Hz) from cholesterol moiety. The presence of this signal showed that no allyl cation rearrangement has occurred during the reaction as was expected.28 Furthermore, we have identified a pattern of signals ascribed to the unchanged carbohydrate moiety such as the methoxyl group (s, 3.5 ppm) and the anomeric H1 (s, 4.9 ppm) which does not couple with H2 because of the dihedral angle (ϕ ∼ 90°) imposed by the pyranoside structure. Carbohydrate H3, however, shows coupling with H2 and H4 (5.9 and 3.8 Hz).
The other pair of dd signals between 2.7 and 2.9 ppm are related to the diastereotopic H6 and H6′, showing gem and cis and trans coupling with H5 (13.0, 8.0 and 3.8 Hz, respectively). The 4.0 ppm multiplet could be easily mistaken with the sterol H3 reactive center at first sight, due to its multiplicity and chemical shift, which would mislead to ascribe it to an axial hydrogen position resultant from mesylate retention of the stereocenter. Since inversion of the steroid C3 stereocenter is expected from a bimolecular substitution of selenium nucleophile, the change of H3 to the equatorial position should produce a more shielded and lower multiplicity signal than the axial alternative. This was indeed observed as a broad signal at 3.5 ppm for the whole series 22–25. Even though the presence of the carbohydrate moiety at the steroid α-face was confirmed by through-space interactions in NOESY experiment. One important observation was made in the phase-sensitive NOE contour map: the hydroxyl hydrogen is ascribed to a doublet signal (d, 2.8 ppm, 3JOH,H5 = 5.0 Hz) coupling with adjacent H5 which has higher multiplicity (m, 3.9 ppm) and interchanges with DHO (1.6 ppm). It is worth mentioning that none of these couplings where observed in the diselenide spectra or for other unprotected glycoconjugated compounds. Fig. 3 depicts the main correlations between hydrogens that help to corroborate the structure.
For the epoxide opening product 6a, NMR structural elucidation was more intricate due to the more complex structure. In this case, the diastereotopism related to H6 observed as a pair of double doublets at 3.1 ppm, common to galactopyranosyl structures, is lost after the glycoconjugation to steroid, turning into a coalesced shielded doublet at 2.7 ppm (Δδ = −0.45 ppm). Even though the other carbohydrate hydrogens are easily identified by COSY. H5 is also more shielded on the steroid glycoconjugated product by comparison with the signal of the same hydrogen in the diselenide (Δδ = −0.11 ppm). The steroid H3 shows the same pattern of multiplicity as the starting materials in the 1H NMR as expected (m, 3.9 ppm) related to the equatorial position and couplings with H4 and H7. Other correlations between the AB fused rings of the steroid moiety were also observed by COSY.
Between 2.1 and 1.6 ppm in the 1H NMR spectra, at least 9 different hydrogen signals are overlapped from both steroid and carbohydrate moieties. However, it was possible to differentiate and assign them to axial or equatorial positions for nuclei connected to the same carbon by HSQC. In contrast to the structure of 25, no phase inverted hydroxyl signal was observed in the NOESY contour map spectra.
Important NOE weak intensity interactions were observed between both carbohydrate and steroid moieties. The steroid alpha selenium H6, observed in the 1H NMR spectra as a broad signal at 2.9 ppm interacts through space with the following carbohydrate hydrogens: anomeric H1 (d, 5.5 ppm), H5 (td, 3.9 ppm) and H6 (d, 2.7 ppm). These interactions helped us to corroborate the regioselectivity of the anti-epoxide nucleophilic ring opening at the sterically less hindered carbon C6 at the steroid beta face. The Fig. 4 depicts the main NOE correlations observed which lead us to assign the carbohydrate moiety on the steroid alpha face.
Conclusions
A method for glycoconjugation by a regioselective epoxide opening or nucleophilic substitution of activated hydroxyls of sterols with different sugar diselenides was developed. The reactions occurred regio and stereoselectively though a trans-diaxial epoxide ring-opening or SN2-substitution, respectively, affording the corresponding sugar conjugated selenosteroids with several carbohydrate moieties.Experimental section
General
1H NMR spectra were recorded either at 300 or 400 MHz and 13C NMR 75 or 101 MHz, respectively with tetramethylsilane as internal standard. High resolution mass spectra were recorded on a Micro-TOF instrument in ESI-mode. Flash column chromatography was performed using silica gel (230–400 mesh) following the methods described by Still.29 Thin layer chromatography (TLC) was performed using supported silica gel GF254, 0.25 mm thickness. For visualization, TLC plates were either placed under ultraviolet light, or stained with iodine vapor, or acidic vanillin or phosphomolybdic acid. THF was dried over sodium benzophenone ketyl and distilled prior to use. DMF was dried over calcium hydride. All other solvents were used as purchased unless otherwise noted. Sugar diselenides were prepared by previously reported methods.16General procedure for the synthesis of steroidal selenoglycoconjugates
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL) were added. The mixture was stirred for 10 minutes at room temperature and the solvents were evaporated under reduced pressure, yielding the corresponding products as a mixture of anomers.
1, 1 mL) were added. The mixture was stirred for 10 minutes at room temperature and the solvents were evaporated under reduced pressure, yielding the corresponding products as a mixture of anomers.Compound 6a
White solid, m.p. 86 °C. [α]20D −79 (c 0.5, CHCl3). 1H NMR (300 MHz, CDCl3): δ = 5.52 (d, J = 5.0, 1H), 4.61 (dd, J = 7.9, J = 2.4, 1H), 4.34 (dd, J = 1.8, J = 7.9, 1H), 4.29 (dd, J = 2.4, J = 5.0, 1H), 4.08–3.98 (m, 1H), 3.93 (dt, J = 1.6, J = 6.9, 1H), 2.91 (d, J = 3.2, 1H), 2.70 (d, J = 6.9, 2H), 2.38–2.98 (m, 2H), 2.07–1.96 (m, 3H), 1.85–1.70 (m, 7H), 1.55 (s, 6H), 1.44 (s, 6H), 1.34 (s, 6H), 1.33 (s, 6H), 1.13–1.17 (m, 10H), 0.91–0.85 (m, 20H), 0.68 (s, 3H). 13C NMR (75 MHz, CDCl3): δ = 109.1, 108.6, 98.5, 77.9, 72.0, 70.8, 70.4, 69.0, 68.0, 56.2, 55.5, 49.0, 46.0, 44.0, 42.7, 39.9, 39.4, 39.1, 36.1, 35.7, 34.6, 32.5, 31.1, 30.7, 28.1, 27.9, 26.2, 26.0, 25.9, 24.9, 24.3, 24.1, 23.8, 22.8, 22.5, 21.2, 18.6, 17.6, 12.1. HRMS-ESI: m/z calcd for C39H66O7Se + Na+: 749.3871, found: 749.3866.Compound 6b
White solid, m.p. 53 °C. [α]20D −75 (c 0.5, CHCl3). 1H NMR (300 MHz, CDCl3): δ = 5.52 (d, J = 5.0, 1H), 5.30 (s, 1H), 4.61 (dd, J = 7.9, J = 2.1, 1H), 4.38 (dd, = 7.9, J = 1.2, 1H), 4.29 (dd, J = 5.0, J = 2.3, 1H), 4.20–4.06 (m, 1H), 3.93 (t, J = 6.9, 1H), 3.60–3.50 (m, 1H), 3.35 (s, 3H), 2.91 (d, J = 2.2, 1H), 2.69 (dd, J = 6.9, J = 2.1, 2H), 2.30–2.22 (m, 1H), 2.04–1.78 (m, 8H), 1.55 (s, 6H), 1.44 (s, 6H), 1.35–1.24 (m, 10H), 1.17–1.18 (m, 10H), 0.91–0.85 (m, 10H), 0.69 (s, 3H). 13C NMR (CDCl3, 75 MHz, ppm) δ = 109.1, 108.5, 96.5, 77.8, 71.8, 70.9, 70.5, 68.8, 56.2, 55.7, 55.5, 49.1, 46.3, 42.7, 40.4, 39.9, 39.4, 39.3, 36.1, 35.7, 34.7, 32.4, 31.1, 28.1, 27.9, 27.0, 26.1, 25.9, 25.9, 24.4, 24.1, 23.8, 22.7, 22.5, 21.2, 21.1, 18.6, 17.5, 12.2. HRMS-ESI: m/z calcd for C40H68O7Se + Na+: 763.4028, found: 763.4022.Compound 7a
White solid, m.p. 113 °C. [α]20D −61 (c 0.5, CHCl3). 1H (CDCl3, 300 MHz, ppm) δ = 5.93 (d, J = 3.5, 1H), 4.54 (d, J = 3.5, 1H), 4.37–4.26 (m, 2H), 4.08–3.96 (m, 2H), 3.02–2.80 (m, 4H), 2.42–2.24 (m, 5H), 2.02–1.73 (m, 5H), 1.51–0.70 (m, 40H). 13C NMR (CDCl3, 75 MHz, ppm) δ = 111.7, 104.6, 85.1, 81.3, 78.0, 74.8, 68.0, 56.4, 55.4, 51.6, 46.0, 44.0, 42.7, 39.8, 39.4, 39.0, 36.1, 35.8, 34.9, 32.4, 31.3, 30.6, 28.2, 28.1, 27.9, 26.7, 26.1, 24.2, 23.9, 22.7, 22.5, 21.0, 18.5, 17.4, 12.3. HRMS-ESI: m/z calcd for C35H60O6Se + Na+: 679.3453, found: 679.3447.Compound 7b
Yellow oil. [α]20D −81 (c 1.0, CH2Cl2). 1H NMR (CDCl3, 300 MHz, ppm) δ = 5.93 (d, J = 3.7, 1H), 4.64–4.27, (m, 1H), 4.36–4.17 (m, 2H), 3.70–3.48 (m, 1H), 3.35 (s, 3H), 3.05–2.64 (m, 4H), 2.29–2.20 (m, 1H), 2.05–1.73 (m, 4H), 1.51–1.44 (m, 4H), 1.33–1.25 (m, 20H), 1.13–1.06 (m, 8H), 0.91–0.85 (m, 10H), 0.69–0.68 (m, 3H). 13C NMR (CDCl3, 75 MHz, ppm) δ = 112.3, 105.5, 85.8, 80.7, 78.4, 76.0, 56.9, 56.5, 56.1, 50.0, 46.9, 43.4, 41.2, 40.6, 40.2, 40.1, 36.8, 36.5, 35.3, 33.0, 32.6, 31.9, 30.4, 28.7, 27.4, 26.8, 24.9, 24.5, 23.8, 23.5, 23.2, 21.9, 19.3, 18.3, 14.8, 12.9. HRMS-ESI: m/z calcd for C36H62O6Se + Na+: 693.3609, found: 693.3604.Compound 8a
Yellow solid, m.p. 60 °C. [α]20D −62 (c 0.5, CHCl3). 1H NMR (300 MHz, CDCl3): δ = 5.02–4.97 (m, 1H), 4.75–4.59 (m, 2H), 4.32–4.27 (m, 1H), 4.10–4.02 (m, 1H), 3.40 (s, 1H), 3.35 (s, 2H), 2.96 (s, 1H), 2.89 (s, 2.87, 1H), 2.80–2.71 (m, 1H), 2.59–2.45 (m, 1H), 2.37–2.11 (s, 1H), 2.04–1.97 (m, 1H), 1.83–1.58 (m, 4H), 1.48 (s, 3H), 1.32–1.24 (m, 5H), 1.15–1.00 (m, 15H), 0.91–0.85 (m, 20H), 0.69 (s, 3H). 13C NMR (CDCl3, 75 MHz, ppm). δ = 133.1, 112.3, 109.57, 86.6, 85.3, 83.7, 77.8, 73.9, 67.9, 56.2, 55.4, 54.9, 53.8, 48.6, 46.0, 44.0, 42.7, 39.9, 39.4, 39.4, 39.1, 36.1, 35.7, 34.6, 32.5, 31.2, 27.9, 26.4, 24.9, 23.8, 22.7, 22.5, 21.2, 18.6, 17.6, 14.5, 12.1. HRMS-ESI: m/z calcd for C36H62O6Se + Na+: 693.3609, found: 693.3604.Compound 8b
Semisolid. [α]20D −49 (c 0.5, CHCl3). 1H NMR (300 MHz, CDCl3): δ = 5.59–5.56 (m, 1H), 5.43–5.27 (m, 2H), 4.97 (s, 3H), 4.71–4.58 (m, 1H), 4.32–4.27 (m, 1H), 4.17–4.11 (m, 1H), 3.60–3.58 (m, 1H), 3.35 (s, 2H), 2.80–2.74 (m, 1H), 2.57–2.53 (m, 1H), 2.31–2.23 (m, 1H), 2.04–1.74 (m, 4H), 1.51–1.25 (m, 7H), 1.11–1.07 (m, 15H), 0.91–0.84 (m, 20H), 0.69 (s, 3H). 13C NMR (CDCl3, 75 MHz, ppm) δ = 133.0, 112.2, 109.6, 86.6, 85.3, 83.6, 77.7, 62.0, 56.2, 55.6, 55.4, 54.9, 48.4, 46.2, 42.7, 40.4, 39.9, 39.4, 36.1, 35.7, 34.8, 32.3, 31.2, 30.1, 28.1, 27.9, 27.0, 26.4, 24.9, 24.1, 23.8, 22.8, 22.5, 21.2, 18.6, 17.5, 12.2. HRMS-ESI: m/z calcd for C37H64O6Se + Na+: 707.3766, found: 707.3760.Compound 9a
Semisolid. [α]22D −9 (c 0.2, CHCl3). 1H NMR (400 MHz, CDCl3): δ = 4.89 (s, 1H), 4.83 (dd, J = 5.9, 3.7 Hz, 1H), 4.57 (d, J = 5.9 Hz, 1H), 4.12–3.96 (m, 1H), 3.85 (dd, J = 8.1, 3.6 Hz, 1H), 3.32 (s, 3H), 3.01–2.86 (m, 3H), 2.77 (dd, J = 13.0, 7.9 Hz, 1H), 2.44–2.29 (m, 2H), 2.08–1.92 (m, 2H), 1.90–1.71 (m, 5H), 1.61–1.05 (m, 26H), 0.90–0.86 (m, 8H), 0.70 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 107.1, 84.8, 81.6, 80.2, 79.8, 79.1, 77.3, 69.3, 68.0, 64.5, 56.3, 55.5, 46.1, 44.0, 39.5, 36.1, 35.8, 35.0, 33.1, 32.6, 31.2, 28.2, 28.0, 26.0, 24.6, 23.8, 22.8, 22.5, 18.6, 17.7, 12.2. HRMS-ESI: m/z calcd for C37H64O7Se + Na+: 723.3715, found: 723.3724.Compound 11a
Colourless oil. [α]22D −94 (c 0.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.52 (d, J = 5.0 Hz, 1H), 4.63–4.57 (m, 2H), 4.33 (dd, J = 7.9, 1.7 Hz, 1H), 4.29 (dd, J = 5.0, 2.4 Hz, 1H), 4.02 (ddd, J = 15.6, 10.5, 5.1 Hz, 1H), 3.94 (td, J = 6.8, 1.2 Hz, 1H), 3.75–3.62 (m, 1H), 2.96–2.86 (m, 2H), 2.70 (d, J = 6.9 Hz, 2H), 2.39–2.33 (m, 1H), 2.21–2.07 (m, 2H), 2.04 (s, 3H), 2.00–1.73 (m, 3H), 1.67–0.87 (m, 28H), 0.82 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 171.2, 109.2, 108.6, 96.6, 82.7, 77.9, 72.1, 70.9, 70.5, 69.1, 68.0, 49.9, 48.9, 46.0, 44.2, 42.8, 39.3, 36.9, 34.3, 32.6, 31.1, 30.7, 27.5, 26.2, 26.2, 26.0, 25.0, 24.4, 23.5, 21.2, 20.7, 17.6, 12.3. HRMS-ESI: m/z calcd for C33H52O8Se + Na+: 679.2725, found: 679.2687.Compound 11b
Colourless oil. [α]22D −171 (c 0.2, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.52 (d, J = 5.0 Hz, 1H), 4.61 (dd, J = 7.9, 2.3 Hz, 1H), 4.34 (dd, J = 7.9, 1.8 Hz, 1H), 4.29 (dd, J = 5.0, 2.4 Hz, 1H), 4.02 (ddd, J = 15.5, 10.4; 5.0 Hz, 1H), 3.96–3.92 (m, 1H), 3.77–3.62 (m, 1H), 2.96–2.88 (m, 2H), 2.70 (d, J = 6.9 Hz, 2H), 2.37–2.31 (m, 1H), 2.07–1.96 (m, 2H), 1.88–1.10 (m, 36H), 0.78 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 109.2, 108.6, 96.5, 77.9, 72.0, 70.9, 70.5, 70.4, 69.0, 68.0, 58.5, 55.0, 49.0, 46.1, 44.1, 42.7, 40.0, 39.2, 34.7, 32.6, 31.1, 30.7, 26.2, 26.1, 26.0, 25.6, 24.9, 24.4, 23.6, 21.1, 17.6, 12.7. HRMS-ESI: m/z calcd for C33H54O8Se + Na+: 681.2882, found: 681.2883.Compound 14
Semisolid. [α]22D −73 (c 0.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.52 (d, J = 5.0 Hz, 1H), 5.15 (dd, J = 15.1, 8.5 Hz, 1H), 5.02 (dd, J = 15.1, 8.6 Hz, 1H), 4.61 (dd, J = 7.9, 2.2 Hz, 1H), 4.35 (dd, J = 7.9, 1.5 Hz, 1H), 4.29 (dd, J = 4.9, 2.3 Hz, 1H), 4.07–3.99 (m, 1H), 3.93 (t, J = 6.9 Hz, 1H), 2.90 (d, J = 3.1 Hz, 1H), 2.70 (d, J = 7.0 Hz, 2H), 2.39–2.28 (m, 1H), 2.07–1.93 (m, 4H), 1.85–1.61 (m, 19H), 1.55 (s, 3H), 1.44 (s, 3H), 1.34 (s, 3H), 1.33 (s, 3H), 1.54–1.06 (m, 10H), 1.02–1.00 (m, 3H), 0.86–0.79 (m, 9H), 0.71 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 138.2, 129.3, 109.3, 108.6, 96.6, 78.0, 72.0, 70.9, 70.5, 69.0, 68.1, 56.0, 55.7, 51.2, 49.1, 46.2, 44.1, 42.7, 40.4, 39.9, 39.2, 34.7, 32.6, 31.8, 31.2, 30.8, 28.8, 26.2, 26.2, 26.0, 25.3, 25.0, 24.4, 24.2, 21.2, 21.2, 21.0, 19.0, 17.7, 12.4, 12.2. HRMS-ESI: m/z calcd for C41H68O7Se + Na+: 775.4028, found: 775.4012.Compound 17
Semisolid. [α]22D −116 (c 0.2, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.52 (d, J = 5.0 Hz, 1H), 4.61 (dd, J = 7.9, 2.3 Hz, 1H), 4.35 (dd, J = 7.9, 1.7 Hz, 1H), 4.29 (dd, J = 5.0, 2.4 Hz, 1H), 4.07–3.99 (m, 1H), 3.93 (td, J = 6.9, 1.3 Hz, 1H), 2.96 (s, 3H), 2.91 (d, J = 3.2 Hz, 1H), 2.88 (s, 3H), 2.71 (d, J = 7.0 Hz, 2H), 2.40–2.27 (m, 1H), 2.06–1.93 (m, 3H), 1.88–1.60 (m, 7H), 1.60 (s, 3H), 1.55 (s, 3H), 1.44 (s, 3H), 1.34 (s, 3H), 1.33 (s, 3H), 1.29–0.98 (m, 14H), 0.92–0.76 (m, 11H), 0.69 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 109.2, 108.6, 96.6, 78.0, 72.0, 70.9, 70.5, 68.9, 68.0, 56.2, 55.6, 49.1, 46.2, 45.8, 44.1, 42.8, 40.0, 39.2, 36.1, 34.7, 33.9, 32.6, 31.2, 30.8, 29.1, 28.2, 26.2, 26.0, 25.0, 24.4, 24.2, 23.0, 21.3, 19.8, 19.0, 18.7, 17.6, 15.4, 12.2, 12.0. HRMS-ESI: m/z calcd for C41H70O7Se + Na+: 777.4184, found: 777.4182.Compound 15
Semisolid. [α]22D −94 (c 0.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.15 (dd, J = 15.2, 8.5 Hz, 1H), 5.02 (dd, J = 15.2, 8.6 Hz, 1H), 4.97 (s, 1H), 4.68 (d, J = 5.9 Hz, 1H), 4.60 (d, J = 5.9 Hz, 1H), 4.29 (dd, J = 9.9, 6.0 Hz, 1H), 4.03 (ddd, J = 15.5, 10.4, 4.9 Hz, 1H), 3.35 (s, 3H), 2.80 (d, J = 3.4 Hz, 1H), 2.76 (dd, J = 12.4, 6.0 Hz, 1H), 2.56 (dd, J = 12.3, 10.1 Hz, 1H), 2.34 (dd, J = 13.3, 11.2 Hz, 2H), 2.10–1.50 (m, 11H), 1.48 (s, 3H), 1.46–1.34 (m, 4H), 1.32 (s, 3H), 1.31–1.11 (m, 10H), 1.11 (s, 3H), 1.01 (d, J = 6.6 Hz, 3H), 0.87–0.76 (m, 9H), 0.71 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 138.2, 129.3, 112.4, 109.6, 86.7, 85.3, 83.7, 77.9, 68.0, 56.0, 55.6, 55.0, 51.2, 48.6, 46.2, 44.2, 42.7, 40.4, 39.8, 39.2, 34.7, 32.6, 31.8, 31.3, 30.8, 30.4, 28.8, 26.5, 25.4, 25.0, 24.2, 21.2, 21.2, 21.1, 19.0, 17.7, 12.4, 12.2. HRMS-ESI: m/z calcd for C38H64O6Se + Na+: 719.3766, found: 719.3767.Compound 18
Yellow oil. [α]22D −363 (c 0.05, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 4.69 (d, J = 5.9 Hz, 1H), 4.60 (d, J = 5.9 Hz, 1H), 4.29 (dd, J = 9.9, 6.0 Hz, 1H), 4.03 (ddd, J = 15.1, 10.3, 4.7 Hz, 1H), 3.35 (s, 3H), 2.80–2.74 (m, 2H), 2.59–2.53 (m, 1H), 2.37–2.31 (m, 1H), 1.85–1.52 (m, 12H), 1.48 (s, 3H), 1.45–1.34 (m, 5H), 1.32 (s, 3H), 1.28–1.15 (m, 11H), 1.11 (s, 3H), 0.99–0.77 (m, 14H), 0.70 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 112.4, 109.6, 86.7, 85.3, 83.7, 77.9, 68.0, 56.2, 55.5, 55.0, 48.6, 46.2, 45.8, 44.2, 42.8, 39.9, 39.2, 36.2, 34.7, 32.6, 31.3, 30.8, 30.4, 29.1, 28.2, 26.5, 26.1, 25.0, 24.2, 23.0, 21.3, 19.8, 19.0, 18.7, 17.6, 15.4, 12.2, 12.0. HRMS-ESI: m/z calcd for C38H66O6Se+: 698.4025, found: 700.4238.Compound 16
Yellow oil. [α]22D −62 (c 0.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.93 (d, J = 3.8 Hz, 1H), 5.15 (dd, J = 15.1, 8.5 Hz, 1H), 5.02 (dd, J = 15.1, 8.6 Hz, 1H), 4.53 (d, J = 3.7 Hz, 1H), 4.35 (ddd, J = 8.4, 5.8, 2.5 Hz, 1H), 4.27 (s, 1H), 4.03 (ddd, J = 15.6, 10.4, 5.1 Hz, 1H), 3.77 (t, J = 5.9 Hz, 1H), 2.95–2.89 (m, 1H), 2.87–2.69 (m, 2H), 2.32 (dd, J = 13.1, 11.3 Hz, 1H), 2.12–1.58 (m, 14H), 1.51 (s, 3H), 1.49–1.33 (m, 6H), 1.31 (s, 3H), 1.28–1.03 (m, 10H), 1.01 (d, J = 6.6 Hz, 3H), 0.87–0.77 (m, 9H), 0.72 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 138.2, 129.3, 111.7, 104.8, 85.1, 80.1, 77.9, 75.4, 68.0, 56.0, 55.6, 51.2, 49.1, 46.1, 44.0, 42.7, 40.4, 39.8, 39.2, 34.6, 32.6, 31.9, 31.3, 30.7, 29.7, 28.8, 26.8, 26.2, 25.4, 24.3, 23.2, 21.2, 21.2, 21.1, 19.0, 17.7, 12.4, 12.2. HRMS-ESI: m/z calcd for C37H62O6Se + Na+: 705.3609, found: 705.3624.Compound 19
Yellow oil. [α]22D −202 (c 0.05, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.93 (d, J = 3.7 Hz, 1H), 4.53 (d, J = 3.7 Hz, 1H), 4.37–4.27 (m, 2H), 4.03 (ddd, J = 15.6, 10.4, 5.0 Hz, 1H), 2.91 (d, J = 3.1 Hz, 1H), 2.81–2.74 (m, 2H), 2.60 (sl, 1H), 2.35–2.29 (m, 1H), 2.06–1.56 (m, 12H), 1.51 (s, 3H); 1.49–1.33 (m, 6H), 1.32 (s, 3H), 1.30–1.12 (m, 10H), 1.10 (s, 3H), 1.03–0.76 (m, 14H), 0.69 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 111.7, 104.8, 85.1, 80.2, 77.9, 75.3, 68.0, 56.2, 55.5, 49.0, 46.0, 45.8, 44.0, 42.8, 39.9, 39.2, 36.1, 34.6, 33.9, 32.5, 31.3, 30.7, 29.1, 28.2, 26.8, 26.2, 26.1, 24.2, 23.1, 23.0, 21.2, 19.8, 19.0, 18.7, 17.7, 15.4, 12.2, 12.0. HRMS-ESI: m/z calcd for C37H64O6Se + Na+: 707.3766, found: 707.3785.Compound 22
Semisolid. [α]22D −53 (c 0.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.53 (d, J = 5.0 Hz, 1H), 5.32 (d, J = 4.9 Hz, 1H), 4.60 (dd, J = 7.9, 2.3 Hz, 1H), 4.39 (dd, J = 7.9, 1.7 Hz, 1H), 4.28 (dd, J = 5.0, 2.3 Hz, 1H), 3.88 (td, J = 7.3, 1.3 Hz, 1H), 3.49 (s, 1H), 2.79 (d, J = 14.3 Hz, 1H), 2.71 (d, J = 7.2 Hz, 2H), 2.22 (d, J = 14.7 Hz, 2H), 2.02–1.73 (m, 6H), 1.68–1.55 (m, 6H), 1.53 (s, 3H), 1.44 (s, 3H), 1.34 (s, 3H), 1.33 (s, 3H), 1.26–1.02 (m, 11H), 1.00 (s, 3H), 0.95 (s, 1H), 0.94–0.89 (m, 4H), 0.87 (d, J = 1.7 Hz, 3H), 0.86 (d, J = 1.7 Hz, 3H), 0.67 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 139.3, 122.2, 109.1, 108.4, 96.7, 71.7, 71.1, 70.6, 68.3, 56.7, 56.1, 49.9, 42.3, 41.6, 39.7, 39.5, 39.0, 37.3, 36.2, 35.8, 35.8, 35.0, 31.8, 28.2, 28.0, 27.7, 26.1, 26.0, 24.9, 24.4, 24.3, 23.8, 22.8, 22.6, 22.5, 20.7, 19.3, 18.7, 11.8. HRMS-ESI: m/z calcd for C39H64O5Se + Na+: 715.3817, found: 715.3795.Compound 23
Yellow oil. [α]22D −37 (c 0.2, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.32 (d, J = 5.0 Hz, 1H), 4.97 (s, 1H), 4.70 (d, J = 5.9 Hz, 1H), 4.60 (d, J = 5.9 Hz, 1H), 4.30 (dd, J = 10.1, 6.0 Hz, 1H), 3.48 (s, 1H), 3.34 (s, 3H), 2.79 (d, J = 14.3 Hz, 1H), 2.73 (dd, J = 12.4, 6.0 Hz, 1H), 2.53 (dd, J = 12.4, 10.2 Hz, 1H), 2.20 (d, J = 14.7 Hz, 1H), 2.02–1.49 (m, 10H), 1.48 (s, 3H), 1.44–1.32 (m, 6H), 1.32 (s, 3H), 1.25–1.04 (m, 10H), 1.00 (s, 3H), 0.91 (d, J = 6.5 Hz, 3H), 0.87 (d, J = 1.8 Hz, 3H), 0.86 (d, J = 1.8 Hz, 3H), 0.67 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 139.1, 122.3, 112.2, 109.6, 86.9, 85.4, 83.7, 56.7, 56.1, 54.9, 50.0, 42.3, 40.8, 39.7, 39.5, 38.8, 37.3, 36.2, 35.8, 34.9, 31.7, 31.7, 28.2, 28.0, 27.6, 26.7, 26.4, 24.9, 24.3, 23.8, 22.8, 22.5, 20.7, 19.3, 18.7, 11.8. HRMS-ESI: m/z calcd for C36H60O4Se + H+: 637.3783, found 637.3696.Compound 24
Semisolid. [α]22D −31 (c 0.05, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.93 (d, J = 3.7 Hz, 1H), 5.32 (d, J = 5.1 Hz, 1H), 4.53 (d, J = 3.8 Hz, 1H), 4.37 (ddd, J = 9.6, 5.2, 2.6 Hz, 1H), 4.28 (dd, J = 5.3, 2.6 Hz, 1H), 3.55 (s, 1H), 2.83 (dd, J = 11.9, 5.3 Hz, 1H), 2.73 (dd, J = 11.9, 9.7 Hz, 1H), 2.21 (d, J = 14.8 Hz, 1H), 2.07 (d, J = 5.5 Hz, 1H), 2.02–1.77 (m, 6H), 1.68–1.52 (m, 6H), 1.50 (s, 3H), 1.46–1.32 (m, 5H), 1.31 (s, 3H), 1.26–1.03 (m, 10H), 1.00 (s, 3H), 0.91 (d, J = 6.5 Hz, 3H), 0.87 (d, J = 1.8 Hz, 3H), 0.86 (d, J = 1.8 Hz, 3H), 0.67 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 139.0, 122.4, 111.6, 104.9, 85.1, 79.9, 75.5, 56.7, 56.1, 50.0, 42.3, 42.0, 39.7, 39.5, 38.8, 37.3, 36.2, 35.8, 35.0, 31.7, 31.7, 28.2, 28.0, 27.7, 26.7, 26.2, 24.3, 23.9, 22.8, 22.5, 20.7, 19.6, 19.3, 18.7, 11.8. HRMS-ESI: m/z calcd for C35H58O4Se + H+: 623.3536, found 623.3335.Compound 25
Semisolid. [α]22D +26 (c 0.2, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ = 5.35 (d, J = 4.6 Hz, 1H), 4.89 (s, 1H), 4.83 (dd, J = 5.8, 3.8 Hz, 1H), 4.56 (d, J = 5.9 Hz, 1H), 3.99 (qd, J = 8.1, 4.4 Hz, 1H), 3.84 (dd, J = 8.1, 3.7 Hz, 1H), 3.50 (sl, 1H), 3.31 (s, 3H), 2.99 (dd, J = 12.9, 3.8 Hz, 1H), 2.85 (d, J = 5.0 Hz, 1H), 2.79 (d, J = 14.0 Hz, 1H), 2.72 (dd, J = 12.9, 8.0 Hz, 1H), 2.25 (d, J = 14.7 Hz, 1H), 2.08–1.48 (m, 13H), 1.47 (s, 3H), 1.46–1.35 (m, 3H), 1.33 (s, 3H), 1.30–1.03 (m, 10H), 1.00 (s, 3H), 0.92 (d, J = 6.5 Hz, 3H), 0.87 (d, J = 6.5 Hz, 6H), 0.67 (s, 3H). 13C NMR (101 MHz, CDCl3): δ = 139.1, 122.4, 112.6, 107.2, 84.8, 81.6, 79.9, 68.5, 56.7, 56.1, 54.6, 50.0, 42.3, 42.1, 39.7, 39.5, 38.8, 37.3, 36.2, 35.8, 35.1, 31.7, 31.7, 29.8, 28.3, 28.2, 28.0, 26.0, 24.6, 24.3, 23.8, 22.8, 22.5, 20.7, 19.3, 18.7, 11.8. HRMS-ESI: m/z calcd forC37H62O5Se + Na+: 689.3660, found: 689.3664.Compound 26
Brown oil. 1H NMR (400 MHz, CDCl3): δ = 5.4–5.3 (m, 1H), 4.8–4.7 (m, 1H), 4.4–4.3 (m, 1H), 3.5–3.2 (m, 2H), 2.91 (s, 1H), 2.5–2.4 (m, 3H), 2.04–0.8 (m, 21H), 0.7 (s, 6H). 13C NMR (101 MHz, CDCl3): δ = 138.4, 123.8, 121.7, 78.7, 56.7, 56.6, 56.1, 50.2, 49.9, 42.7, 42.3, 39.6, 39.5, 37.4, 36.7, 36.1, 35.8, 31.9, 31.8, 29.7, 28.2, 28.0, 27.2, 24.2, 23.8, 22.8, 22.5, 21.0, 19.4, 19.2, 18.7, 18.6, 11.8. HRMS-ESI: m/z calcd for C33H58O7Se + H+: 647.3429, found 647.3348.Compound 27
Brown oil. 1H NMR (400 MHz, CDCl3): δ = 5.4–5.3 (m, 1H), 4.8–4.7 (m, 1H), 4.1–4.0 (m, 1H), 3.6–2.8 (m, 3H), 2.4–0.9 (m, 44H), 0.7 (s, 6H). 13C NMR (101 MHz, CDCl3): δ = 140.6, 138.4, 123.7, 121.7, 78.7, 71.7, 56.7, 56.6, 56.1, 55.8, 49.8, 42.7, 42.1, 39.5, 39.3, 37.4, 36.6, 36.0, 35.4, 31.8, 31.6, 29.6, 28.2, 27.9, 27.2, 24.2, 23.4, 22.7, 22.3, 21.1, 18.6, 11.7. HRMS-ESI: m/z calcd for C32H56O6Se + H+: 617.3323, found 617.3325.Compound 28
Brown oil. 1H NMR (400 MHz, CDCl3): δ = 5.39–5.32 (m, 1H), 4.89–4.83 (m, 1H), 4.57–4.52 (m, 1H), 4.07–3.84 (m, 4H), 3.49–3.37 (m, 3H), 3.34–2.81 (m, 5H), 2.39–0.65 (m, 43H). 13C NMR (101 MHz, CDCl3): δ = 132.4, 128.9, 126.9, 125.0, 123.1, 56.9, 56.1, 50.1, 48.4, 43.5, 42.4, 42.3, 40.9, 40.3, 39.8, 39.5, 36.2, 35.8, 34.0, 33.8, 31.8, 28.3, 28.2, 28.0, 27.6, 27.5, 24.3, 23.8, 22.8, 22.7, 22.5, 21.5, 18.7, 12.0. HRMS-ESI: m/z calcd for C34H58O5Se + H+: 627.3531, found 627.3522.Notes and references
- B. Heasley, Chem.–Eur. J., 2012, 18, 3092 CrossRef CAS PubMed.
- (a) M. Slingerland, C. Cerella, H. J. Guchelaar, M. Diederich and H. Gelderblom, Invest. New Drugs, 2013, 31, 1087 CrossRef CAS PubMed; (b) R. A. Newman, P. Yang, A. D. Pawlus and K. I. Block, Mol. Interventions, 2008, 8, 36 CrossRef CAS PubMed; (c) J. A. R. Salvador, J. F. S. Carvalho, M. A. C. Neves, S. M. Silvestre, A. J. Leitão, M. C. Silva and M. L. Sá e Melo, Nat. Prod. Rep., 2013, 30, 324 RSC.
- (a) M. Jensen, S. Schmidt, N. U. Fedosova, J. Mollenhauer and H. H. Jensen, Bioorg. Med. Chem., 2011, 19, 2407 CrossRef CAS PubMed; (b) H. A. Elbaz, T. A. Stueckle, H.-Y. L. Wang, G. A. O'Doherty, D. T. Lowry, L. M. Sargent, L. Wang, C. Z. Dinu and Y. Rojanasakul, Toxicol. Appl. Pharmacol., 2012, 258, 51 CrossRef CAS PubMed.
- (a) J. M. Langenhan, N. R. Peters, I. A. Guzei, F. M. Hoffmann and J. S. Thorson, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12305 CrossRef CAS PubMed; (b) M. Lopez-Lazaro, N. Pastor, S. S. Azrak, M. J. Ayuso, C. A. Austin and F. Cortes, J. Nat. Prod., 2005, 68, 1642 CrossRef CAS PubMed.
- L. J. Rashan, K. Franke, M. M. Khine, G. Kelter, H. H. Fiebig, J. Neumann and L. A. Wessjohann, J. Ethnopharmacol., 2011, 134, 781 CrossRef CAS PubMed.
- C. Bobach, S. Tennstedt, K. Palberg, A. Denkert, W. Brandt, A. de Meijere, B. Seliger and L. A. Wessjohann, Eur. J. Med. Chem., 2015, 90, 267 CrossRef CAS PubMed.
- (a) K. Pérez-Labrada, I. Brouard, S. Estévez, M. T. Marrero, F. Estévez and D. G. Rivera, Bioorg. Med. Chem., 2012, 20, 4522 CrossRef PubMed; (b) K. Pérez-Labrada, I. Brouard, S. Estévez, M. T. Marrero, F. Estévez, J. Bermejo and D. G. Rivera, Bioorg. Med. Chem., 2012, 20, 2690 CrossRef PubMed.
- Y. Wang, Y. Zhang, Z. Zhu, S. Zhu, Y. Li, M. Lia and B. Yua, Bioorg. Med. Chem., 2007, 15, 2528 CrossRef CAS PubMed.
- (a) R. M. Phan and C. D. Poulter, J. Org. Chem., 2001, 66, 6705 CrossRef CAS PubMed; (b) T. A. Lin, O. Boutureira, L. Lercher, B. Bhushan, R. S. Paton and B. G. Davis, J. Am. Chem. Soc., 2013, 135, 12156 CrossRef PubMed.
- (a) K. Pérez-Labrada, I. Brouard, C. Morera, F. Estévez, J. Bermejo and D. G. Rivera, Tetrahedron, 2011, 67, 7713 CrossRef; (b) A. M. Deobald, L. R. S. Camargo, D. Alves, J. Zukerman-Schpector, A. G. Corrê and M. W. Paixão, Synthesis, 2011, 24, 4003 Search PubMed.
- D. G. Rivera, K. Pérez-Labrada, L. Lambert, S. Dorner, B. Westermann and L. A. Wessjohann, Carbohydr. Res., 2012, 359, 102 CrossRef CAS PubMed.
- L. A. Wessjohann, A. Schneider, M. Abbas and W. Brandt, Biol. Chem., 2007, 10, 997 Search PubMed.
- (a) H. C. Braga, H. A. Stefani, M. W. Paixão, F. W. Santos and D. S. Lüdtke, Tetrahedron, 2010, 66, 3441 CrossRef CAS; (b) J. P. Vargas, L. M. Pinto, L. Savegnago and D. S. Lüdtke, J. Braz. Chem. Soc., 2015, 26, 810 CAS.
- H. C. Braga, A. D. Wouters, F. B. Zerillo and D. S. Lüdtke, Carbohydr. Res., 2010, 345, 2328 CrossRef CAS PubMed.
- A. L. Braga, W. A. Severo-Filho, R. S. Schwab, O. E. D. Rodrigues, L. Dornelles, H. C. Braga and D. S. Lüdtke, Tetrahedron Lett., 2009, 50, 3005 CrossRef CAS.
- R. F. Affeldt, H. C. Braga, L. L. Baldassari and D. S. Lüdtke, Tetrahedron, 2012, 68, 10470 CrossRef CAS.
- (a) S. Wollenhaupt, A. T. Soares, W. G. Salgueiro, S. Noremberg, D. Bohrer, P. Gubert, F. A. Soares, R. F. Affeldt, D. S. Lüdtke, F. W. Santos, C. C. Denardin, M. Aschner and D. S. Avila, Food Chem. Toxicol., 2014, 64, 192 CrossRef CAS PubMed; (b) L. M. Vargas, M. B. Soares, A. P. Izaguirry, D. S. Lüdtke, H. C. Braga, L. Savegnago, S. Wollenhaupt, D. S. Brum, F. G. Leivas and F. W. Santos, J. Appl. Toxicol., 2013, 33, 679 CrossRef CAS PubMed.
- C. C. Spiazzi, M. B. Soares, A. P. Izaguirry, L. M. Vargas, M. Zanchi, N. F. Pavin, R. F. Affeldt, D. S. Lüdtke, M. Prigol and F. W. Santos, Oxid. Med. Cell. Longevity, 2016, 65, 976908, DOI:10.1016/j.pnpbp.2015.10.008.
- (a) R. Strommer, C. Hödl, W. Strauss, R. Sailer, E. Haslinger, H. W. Schramm and C. Seger, Monatsh. Chem., 2004, 135, 1137 CrossRef CAS; (b) H.-S. Kim and J.-H. Kang, Bull. Korean Chem. Soc., 2001, 22, 1390 CAS; (c) E. Ma and T. Choi, Bull. Korean Chem. Soc., 2009, 30, 245 CrossRef CAS.
- (a) S. D. Sarkar, R. D. Ice, W. H. Beierwaltes, S. P. Gill, S. Balachandran and G. P. Basmadjian, J. Nucl. Med., 1976, 17, 212 CAS; (b) S. A. Sadek, S. M. Shaw, W. V. Kessler and G. C. Wolf, J. Org. Chem., 1981, 46, 3259 CrossRef CAS.
- T. E. Frizon, J. Rafique, S. Saba, I. H. Bechtold, H. Gallardo and A. L. Braga, Eur. J. Org. Chem., 2015, 16, 3470 CrossRef.
- O. E. D. Rodrigues, D. Souza, L. C. Soares, L. Dornelles, R. A. Burrow, H. R. Appelt, C. F. Alves, D. Alves and A. L. Braga, Tetrahedron Lett., 2010, 51, 2237 CrossRef CAS.
- M. H. M. Sari, A. C. G. Souza, S. G. Rosa, D. Souza, O. E. D. Rodrigues and C. W. Nogueira, Eur. J. Pharmacol., 2014, 725, 79 CrossRef PubMed.
- (a) E. Ma, H. Kim and E. Kim, Steroids, 2005, 70, 245 CrossRef CAS PubMed; (b) E. Ma and S. Kim, Molecules, 2009, 14, 4655 CrossRef PubMed; (c) C. M. Marson, A. S. Rioja, K. E. Smith and J. M. Behan, Synthesis, 2003, 535 CrossRef CAS; (d) D. A. Foley, Y. O'Callaghan, N. M. O'Brien, F. O. McCarthy and A. R. Maguirre, J. Agric. Food Chem., 2010, 58, 1165 CrossRef CAS PubMed.
- The same behaviour was previously observed by our group, see ref. 14.
- (a) R. A. Moreau, B. D. Whitaker and K. B. Hicks, Prog. Lipid Res., 2002, 41, 457 CrossRef CAS PubMed; (b) J. Quílez, P. García-Lorda and J. Salas-Salvado, Clin. Nutr., 2003, 22, 343 CrossRef; (c) D. Kritchevsky and S. C. Chen, Nutr. Res., 2005, 25, 413 CrossRef CAS; (d) G. Brufau, M. A. Canela and M. Rafecas, Nutr. Res., 2008, 28, 217 CrossRef CAS PubMed; (e) G. García-Llatas and M. T. Rodríguez-Estrada, Chem. Phys. Lipids, 2011, 164, 607 CrossRef PubMed.
- (a) S.-S. Huang, K.-L. Jian, R.-J. Li, L.-Y. Kong and M.-H. Yang, RSC Adv., 2016, 6, 6320 RSC; (b) X. Lin, L. Ma, R. A. Moreau and R. E. Ostlund Jr, Lipids, 2011, 8, 701 CrossRef PubMed; (c) X. Lin, L. Ma, S. B. Racette, C. L. A. Spearie and R. E. Ostlund Jr, Am. J. Physiol. Gastrointest. Liver Physiol., 2009, 296, 931 CrossRef PubMed; (d) A. Waheed, J. Barker, S. J. Barton, C. P. Owen, S. Ahmed and M. A. Carew, Eur. J. Pharm. Sci., 2012, 47, 464 CrossRef CAS PubMed.
- Q. Sun, S. Cai and B. R. Peterson, Org. Lett., 2009, 11, 567 CrossRef CAS PubMed.
- W. C. Still, M. Kahn and A. Mitra, J. Org. Chem., 1978, 43, 2923 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization data and copies of NMR spectra. See DOI: 10.1039/c6ra21485a |
| This journal is © The Royal Society of Chemistry 2016 |