Preparation of honokiol with biodegradable nanoparticles for treatment of osteosarcoma
Abstract
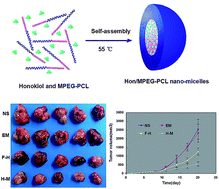
In previous studies, honokiol (Hon) has been demonstrated to have anti-tumorigenic effects. However, its poor water solubility and low bioavailability limit its potential as an orally-administered anti-cancer compound. This study presents work toward improving the water solubility and antitumor effects of honokiol through the use of self-assembling, biodegradable micelles of monomethoxy poly(ethylene glycol)–poly(ε-caprolactone) copolymer (MPEG–PCL). These honokiol-containing polymeric micelles (Hon/MPEG–PCL) release the compound slowly over an extended period of time. In addition, treatment with Hon/MPEG–PCL inhibits the growth of osteosarcoma cells and induces apoptosis of these cells more effectively than free honokiol in vitro. Further, in a nude mouse model of osteosarcoma, Hon/MPEG–PCL inhibits the growth of tumors, induces apoptosis, and inhibits angiogenesis more effectively than the same dose of free honokiol. This shows that Hon/MPEG–PCL enhances the antitumor effects of honokiol in vivo, and, as a result, shows great promise as a clinical treatment for osteosarcoma.

 Please wait while we load your content...
Please wait while we load your content...