Rumen tissue derived decellularized submucosa collagen or its chitosan-treated film as a cutaneous wound healant and 1H NMR-metabolite profiling of plasma
K. Gopal Shankara,
S. Udhaya Kumara,
S. Sowndaryaa,
J. Sridevib,
S. Soniya Angela and
C. Rose*a
aBiochemistry and Biotechnology Laboratory, CSIR-Central Leather Research Institute, Adyar, Chennai 600 020, India. E-mail: rose@clri.res.in; chellanrose@yahoo.co.uk; Tel: +91-44-24430273
bInorganic & Physical Chemistry Laboratory, CSIR-Central Leather Research Institute, Chennai 600 020, India
First published on 27th October 2016
Abstract
Developing an ideal wound dressing material for skin defects is of significant importance in a clinical emergency and is currently a global burden. Decellularized extracellular matrix derivatives from various xenogeneic origins have been successfully used as dermal substitutes, since they contain perpetuated bioactive molecules that serve as a regenerative template for faster dermal reconstruction. The major objective of this study is to evaluate the in vivo biocompatibility of an un-tapped type I collagen film (COL-F) derived from bovine rumen submucosa, in comparison to the same COL-F treated with 1% (w/v) chitosan solution to produce another film, COL/CS-F. The comparative study includes the evaluation of physical and biological properties of the films in treating open excision wounds in rats. Both COL-F and COL/CS-F were observed to possess excellent fluid uptake ability, good water retention and controlled degradation kinetics. The topical application of the films resulted in exquisite wound healing on day 16, with wound contraction rates of 92.83% ± 0.95% and 94.83% ± 0.46% for COL-F and COL/CS-F, respectively. The estimation of biochemical parameters (collagen, hexosamine and uronic acid) was correlated with histological observations, which elucidate the evidence for effective collagen synthesis and tissue vascularisation as a result of better wound prognosis. The metabolomics of the wound healing phase was acquired by nuclear magnetic resonance (NMR) spectroscopy, which demonstrated time-dependent up and down regulation of metabolites in response to injury. Overall, the presence of chitosan in the COL-F accelerated wound healing by exerting relevant biomimetic and chemotactic effects at the wound surface. The ability of COL-F and COL/CS-F to treat open wounds with favourable tissue re-epithelization and remodelling at a shorter duration demonstrates its potential feasibility in the field of skin tissue engineering.
1. Introduction
With the skin being the largest organ of the body, the loss of skin due to trauma, burns and chronic diseases has become one of the most serious clinical problems. Globally, every year more than 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people die as a result of burn injuries.1 The disruption of normal skin physiology leads to dermal injuries and the healing mechanism is a complex process where different cell types and growth factors associate to re-build the skin continuity.2,3 The reconstruction of the damaged tissue occurs in an imbricated series of events, which involves the inflammation, proliferation and remodelling phases.4 The initiation of the clotting cascade at the injury site results in the proteolytic cleavage of fibrinogen by the enzyme thrombin, thus forming an insoluble fibrin clot that holds damaged tissues and forms the amorphous extra cellular matrix (ECM). This matrix is devoted to the formation of granulation tissue by producing a scaffold or conduit for the migration and activation of fibroblasts.5,6
000 people die as a result of burn injuries.1 The disruption of normal skin physiology leads to dermal injuries and the healing mechanism is a complex process where different cell types and growth factors associate to re-build the skin continuity.2,3 The reconstruction of the damaged tissue occurs in an imbricated series of events, which involves the inflammation, proliferation and remodelling phases.4 The initiation of the clotting cascade at the injury site results in the proteolytic cleavage of fibrinogen by the enzyme thrombin, thus forming an insoluble fibrin clot that holds damaged tissues and forms the amorphous extra cellular matrix (ECM). This matrix is devoted to the formation of granulation tissue by producing a scaffold or conduit for the migration and activation of fibroblasts.5,6
The global perception of wound healing has expanded immensely with recent advances in skin tissue engineering. The inception of wound healing is often guided by the use of biocompatible wound dressing materials. In the past decades, numerous dermal substitutes have been developed and applied for the treatment of full thickness skin defects. For example, Alloderm®, an acellular allograft dermal matrix and Integra® Dermal Regeneration Template, a bi-layer dermal substitute, are some of the products available commercially.7,8 Similarly, there are several artificial polymers, such as poly(lactic acid), poly(glycolic acid) and their co-polymer poly(lactic-co-glycolic acid) (PLGA) and PLGA knitted and mesh-reinforced collagen–chitosan scaffold.9,10 These materials have been demonstrated to provide sufficient internal connective space for tissue in growth and also serve as a “skeleton” to reinforce collagen-based scaffolds.11,12 Among the natural biopolymers, collagen–chitosan scaffolds are considered to be suitable biomaterials for skin tissue engineering owing to their appreciable physical, chemical and biological characteristics.13,14 Collagen being a major constituent of connective tissue and chitosan being a pro-fuser molecule in nature, upon processing, the final composite will act as bio-adhesive material, thereby increasing its retention at the application site.6,15,16 Accordingly, there are several studies that report the potential properties of collagen–chitosan scaffolds with physical and chemical modifications, in order to develop a tailor made scaffold for regenerative medicine.17–21
The use of decellularized connective tissue matrices in tissue engineering is in high demand, due to their biomimetic properties and clinical suitability as substitutes in skin tissue engineering. The potency of these ECM derived scaffolds in clinical use is due to the presence of a concoction of bioactive molecules, such as collagen, fibronectin, laminin, glycosaminoglycans (GAGs), hyaluronic acid, growth factors and cytokines, which enhance the wound healing cascade.22 For example, small intestine submucosa (SIS) has the native collagen type (I) and type (IV) layered into sheets, and it is commercially available for soft tissue regeneration.23 SIS sponges cross-linked with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) were reported to provide enhanced healing of full thickness skin wounds in rats, due to their excellent fluid absorption capacity.24 Cholecyst-derived scaffolds (CDS) from porcine gall bladder was used as a skin graft to heal full thickness wounds in a rabbit model.25 The encouraging results from these in vivo studies have manifested that the intact native ECM structures are more suitable for cell colonisation that helps in the constructive remodelling of wounds.
In this paper, we report the in vivo biocompatibility of collagenous films derived from a new source – the bovine rumen submucosa layer. In our previous work we reported the method of isolation, separation and decellularization of the bovine rumen submucosa layer to produce fibro-collagenous film and their physico-chemical and mechanical characterization, along with the in vitro biocompatibility test.26 This study aims to use the collagenous film of this tissue, with the incorporation of chitosan, to develop a hybrid bio-composite for comparison. Chitosan, being a reinforcement material, exhibits superior characteristics such as bacteriostatic and fungistatic activities.27 The major objective of this research work is to evaluate the in vivo efficacy of this fibro-collagenous film to treat open excision wounds in the rat model through the qualitative and quantitative assessment of bio-chemical parameters pertaining to the wound healing response. More importantly, this study aims to monitor the metabolism during the course of healing by following the blood metabolomics response through NMR spectral measurements.
2. Materials and methods
2.1 Chemicals and reagents
Pepsin, chitosan (85% deacetylated), lysozyme, trichloroacetic acid (TCA), (5,5′-dithiobis-(2-nitrobenzoic acid)) – (DTNB) reagent, dinitrophenylhydrazine (DNPH) reagent, thiourea-copper sulphate (DTC) reagent, glutathione reductase, ascorbic acid, hydroxyproline, glucosamine HCl and D-glucuronic acid lactone were purchased from Sigma-Aldrich, (USA). Standard nutrient broth and antibiotic and antimitotic solution were purchased from Hi-Media Laboratories, (India). All other chemicals and reagents used were of analytical grade or molecular grade, purchased from SRL chemicals, (India).2.2 Preparation of collagen and collagen–chitosan films
Collagen film derived from bovine rumen submucosa was prepared according to the method of Shankar et al.26 Briefly, the tunica muscularis layer of the rumen tissue, along with the luminal layer was removed and the submucosa layer was subjected to successive treatments with 2% (w/v) calcium hydroxide solution for 6–8 h at 4 °C, followed by washing with isopropyl alcohol and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The obtained tissue layer was subjected to pepsin treatment (1
1 v/v). The obtained tissue layer was subjected to pepsin treatment (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000 from porcine stomach mucosa), 1
3000 from porcine stomach mucosa), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (w/w), pH 2.0, at 4 °C for 3–5 h, after which the matrix was soaked in 0.2 M disodium hydrogen phosphate to regain the native status of collagen fibres in the matrix. Later, the tissue was disinfected using 2% (w/v) sodium metabisulfite (SMBS) and washed in sterile water to remove the residual salts and air dried at 25 °C. The air-dried samples thus obtained appeared white in colour and are referred to as collagen film (COL-F).
100 (w/w), pH 2.0, at 4 °C for 3–5 h, after which the matrix was soaked in 0.2 M disodium hydrogen phosphate to regain the native status of collagen fibres in the matrix. Later, the tissue was disinfected using 2% (w/v) sodium metabisulfite (SMBS) and washed in sterile water to remove the residual salts and air dried at 25 °C. The air-dried samples thus obtained appeared white in colour and are referred to as collagen film (COL-F).
Collagen–chitosan film (COL/CS-F) was prepared by treating the COL-F with 1% (w/v) chitosan solution in 0.5 M acetic acid for 12–16 h at 4 °C. The chitosan-treated COL-F thus prepared was air dried at 25 °C and was referred to as COL/CS-F. Both COL-F and COL/CS-F were sterilized by exposing the samples to 80% ethylene oxide atmosphere at a relative humidity of 50% at (33 °C) for 16 h. After sterilization, the samples were aerated for at least 48 h to remove residual ethylene oxide from the films before being used in further experiments.
2.3 Moisture content in the films
The presence of moisture within the films was estimated in order to assimilate the hydration abilities of the films. Briefly, the films were cut into sizes of approximately 1 cm2 and the initial weight was noted. The films were placed in an oven at 40 °C for 24 h and later, the dry weight of the films was recorded. The percentage moisture content was calculated as [(initial weight − dry weight) × 100]/initial weight. Three samples in each group were analysed.2.4 Swelling ability
The ability of the films to uptake water under physiological conditions was determined by assessing the swelling behaviour of the individual samples. Briefly, the films were cut into sizes of approximately 1 cm2 and the initial weights were noted. All samples were immersed in phosphate buffered saline (PBS) at pH 7.4 at 37 °C. After 1, 3, 6, 12, 24 h, the films were taken separately, blotted in filter paper and the wet weights were noted. The percentage swelling ability was calculated as [(wet weight) − (dry weight) × 100]/(dry weight). Three samples in each group were analysed.2.5 In vitro enzymatic degradation
The biodegradation test on the films was carried out by the method of Tangsadthakun et al.28 All samples were immersed in PBS (pH 7.4) solution containing 0.5 mg mL−1 of lysozyme at 37 °C for 21 days. The enzymatic solution was changed every 3 days to provide maximum enzyme activity during the study. After 7, 14 and 21 days, the respective samples were taken and freeze dried, and the final dry weights were noted. The percentage weight loss was calculated as (initial dry weight − final dry weight)/(initial dry weight) × 100. Three samples in each group were analysed.2.6 Water vapour transmission rate
The moisture permeability of the films was assessed by measuring the water vapour transmission rate (WVTR) following the method of Revi et al.25 The films were cut into sizes of approximately 1 cm2, a 15 mL plastic tube was filled with 10 mL of distilled water and the mouth of the tube was sealed by using the film and the initial weight was noted. The tubes were also tape casted to ensure complete closure of the tube mouth with the films to avoid unwanted water loss. The tubes sealed with the film(s) were placed in an incubator at 37 °C for 24 h. Later, the weight after the evaporation of water from the tubes was noted. The WVTR was calculated as [(initial weight − weight after evaporation) × 106]/A × 24. ‘A’ denotes the area of the mouth of the tube in mm2 and WVTR is expressed in g mm−2 h−1. Three samples in each group were analysed.2.7 Microbial penetration test
The ability of the films to inhibit microbial penetration was assessed by attaching the films on top of a glass tube containing 20 mL of standard nutrient broth. Prior to attaching the films, all the samples were sterilized as mentioned earlier and the nutrient broth and glass test tubes were sterilized by autoclaving at 121 °C for 30 min. The negative control was sterile nutrient broth in a glass test tube closed with a cotton ball and sealed with paraffin film, whereas the positive control was sterile nutrient broth in a glass test tube exposed to air at 30 °C. After 7 days of incubation, the cloudiness of the nutrient broth in the glass test tube was considered as microbial contamination. The spectrophotometric measurements at 600 nm were carried out using a micro plate reader (Thermo Scientific Multiskan Ascent, USA). Three samples in each group were analysed.2.8 In vivo studies – rat excision wound model
Male albino rats were purchased from Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), Chennai, India, and were maintained under hygienic conditions in individual metabolic cages. They were provided with a commercial balanced diet and water ad libitum. All experiments were performed in accordance with the rules and regulations of the Indian Animal Ethics Committee (IAEC) vide approval no. 466/01/a/CPCSEA dated: 13-8-2012.2.9 Wound creation and treatment
A total of 36 male albino rats weighing 285 ± 7 g were separated into 3 groups: control (untreated, n = 12), COL-F treated (n = 12) and COL/CS-F treated (n = 12). All animals were anesthetized with ketamine (100 mg kg−1 body weight) through intraperitoneal injection. The surgical area was shaved using a sterile blade to remove the dorsal fur and was sterilized using 70% ethanol. In all the rats a full thickness excision wound measuring 1.5 cm long × 1.5 cm wide was created below the cervical region using standard surgical blades. In the control group, the wound was left empty and only sterile PBS (pH 7.4) was added. In the experimental groups, sterile PBS (pH 7.4), pre-wetted COL-F and COL/CS-F of size 1.5 × 1.5 cm2 were topically applied over the wound and 3M micro-pore tape (3M™, USA) was used to hold the edges of the wounded skin so as to ensure that the four sides of the films were in contact with the excision wound. Additionally, sterile absorbent gauze was placed over the films and dressing was carried out at a regular interval of 4 days with respective films. The healing pattern of all wounds was assessed at regular time intervals of 4, 8 and 16 days of the experimental period. On the day of tissue collection, the individual animals were first subjected to mild ether anesthesia and later euthanized by cervical dislocation, and the granulation tissue was collected and stored at −80 °C until analysis.2.10 Wound planimetry
The rate of wound healing was assessed periodically in both the control and experimental wounds by measuring the contour of the wound using a transparent graph sheet, according to the method reported by Morgen et al.29 The rate of epithelialisation was calculated and it is expressed as percentage contraction. Percentage of wound contraction = [(wound area on day 1 − wound area on day n) × 100]/wound area on day 1.2.11 Biochemical analysis of tissue
The granulation tissues collected at regular time intervals were subjected to the estimation of collagen and hexosamine by the methods of Woessner30 and Elson et al.,31 respectively. The production of uronic acid in the granulation tissue was analysed by the method of Bitter et al.32 and measured by the method of Schiller et al.33 The soluble protein content in the re-epithelialized tissue was measured by the method of Lowry et al.34 All the parameters were analysed in triplicate and read spectrophotometrically between 500-700 nm using a micro plate reader (Thermo Scientific Multiskan Ascent, USA).2.12 Estimation of non-enzymatic antioxidants
The levels of reduced glutathione in the re-epithelialized tissue specimens were estimated by the method of Moron.35 Briefly, 1 mL of skin homogenate supernatant was precipitated with 5% TCA and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The resultant supernatant was added to 0.5 mL of PBS (pH 8.0), followed by the addition of 2 mL DTNB reagent. The spectrophotometric measurements at 412 nm were carried out using a micro plate reader (Thermo Scientific Multiskan Ascent, USA). A standard glutathione solution of known concentration was similarly estimated and the amount of glutathione was calculated and expressed as μg mg−1 protein. Three samples in each group were analysed.
000 rpm for 10 min. The resultant supernatant was added to 0.5 mL of PBS (pH 8.0), followed by the addition of 2 mL DTNB reagent. The spectrophotometric measurements at 412 nm were carried out using a micro plate reader (Thermo Scientific Multiskan Ascent, USA). A standard glutathione solution of known concentration was similarly estimated and the amount of glutathione was calculated and expressed as μg mg−1 protein. Three samples in each group were analysed.
Similarly, the levels of ascorbic acid were estimated by the method of Omaye et al.36 Briefly, the supernatant from the skin homogenate was precipitated using ice-cold TCA and centrifuged at 3500 g for 20 min. Following this, 0.2 mL of DNPH–DTC reagent was added to the 1 mL supernatant and incubated at 37 °C for 3 h. Later, 1.5 mL of ice-cold 65% H2SO4 was added and mixed well and further incubated at 37 °C for 30 min. The spectrophotometric measurements at 520 nm were carried out using a micro plate reader (Thermo Scientific Multiskan Ascent, USA). A series of varied concentrations of ascorbic acid was used as standard and the amount of ascorbic acid present in the tissue was calculated and expressed as μg mg−1 protein. Three samples in each group were analysed.
2.13 Histology
Immediately after animal euthanization, the tissues from the wounded sites were collected and fixed in 0.1 M phosphate buffer (pH 7.4) containing 10% formalin for 72 h. The specimens were then cassetted and embedded in paraffin and sectioned using a rotary microtome (Leica RM 2125RTS, Germany). Sections of 5 μm thickness were stained with Hematoxylin and Eosin (H&E) and Masson Trichrome (MT) dye to evaluate the histological and histochemical features of the tissue.37 All the stained sections were cover slipped and viewed under trinocular bright field microscope (LEICA-DM IL LED, Germany).2.14 NMR studies
At respective time-points, immediately before animal euthanization under terminal anesthesia, 5 mL of blood sample were collected through cardiac puncture in anticoagulant (EDTA)-treated tubes. The blood was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and 1 mL of supernatant was stored at −20 °C until analysis. Plasma samples were defrosted at room temperature, 350 μL of plasma was mixed with 150 μL of deuterium oxide (D2O) and transferred into a 5 mm OD NMR sample tube. 1H-NMR acquisition was carried out in a 400 MHz high resolution BRUKER-Ascend TM 400 NB NMR spectrometer (Switzerland), with the following experimental parameters: 5 mm PABBO BB probe and zg30 pulse program with 128 scans. The FID data was processed using BRUKER Topspin (3.2) software.
000 rpm for 15 min and 1 mL of supernatant was stored at −20 °C until analysis. Plasma samples were defrosted at room temperature, 350 μL of plasma was mixed with 150 μL of deuterium oxide (D2O) and transferred into a 5 mm OD NMR sample tube. 1H-NMR acquisition was carried out in a 400 MHz high resolution BRUKER-Ascend TM 400 NB NMR spectrometer (Switzerland), with the following experimental parameters: 5 mm PABBO BB probe and zg30 pulse program with 128 scans. The FID data was processed using BRUKER Topspin (3.2) software.
2.15 Statistical analysis
Results were expressed as mean ± SEM plotted on graph. Statistical analysis was performed by the Graph Pad Prism software (version 6.0), with statistical significance set at P ≤ 0.05.3. Results and discussion
The selection of appropriate healing material is significantly important, as it greatly influences and determines the rate of healing. Accordingly, the physical, chemical and biological composition of the dressing material are considered to be highly indispensable, as these properties can arbitrate to the functional outcomes of the healing pattern.38–40 The isolation, preparation, characterization and biocompatibility of COL-F and COL/CS-F were reported in our previous publication.26 The surface morphology of COL-F exhibited a fibrous architecture with random collagenous networking and COL/CS-F appeared to be smooth, due to chitosan coating. The addition of chitosan in COL-F increased the tensile properties, as well as the thermal stability and durability of the films. The presence of respective amide peaks, which corresponds to protein (collagen) and the evidence of collagen–chitosan interaction was observed through infrared spectroscopic analysis. The submucosa layer was electrophoretically found to have native type I collagen and the presence of amorphous and crystalline peaks, attributed to the triple helical structure of collagen in the films, was evident from X-ray diffraction studies. The results of cell viability and proliferation demonstrated that COL-F and COL/CS-F exhibit good biocompatibility and can therefore augment cell infiltration and proliferation.26 These encouraging and promising results highly influenced the need to study the biocompatibility of these films in a suitable animal model. Accordingly, in this study, the use of ECM derived natural COL-F and COL/CS-F films from bovine rumen have been studied to investigate the physical characteristics and also to evaluate the potency of the films in healing cutaneous wounds in the rat model.3.1 Moisture content
The presence of residual moisture content in COL-F (8.2 ± 0.77%) and COL/CS-F (12.2 ± 1.5%) is illustrated in Fig. 1A, with statistical difference between the two groups (p < 0.05). This shows that the incorporation of chitosan into the COL-F has helped in retaining more moisture within its matrix, due to the hydrophilic nature of chitosan, thereby allowing the absorption of water molecules within its intra-molecular linkages. Maintaining the hydrated state in the ECM scaffolds throughout the entire period of decellularization and sterilization is highly essential for the cellular activity at the wound site because insufficient water can cause the collapse of collagen fibres within the matrix, which can lead to the formation of physical bonds between the ECM molecules, which apparently affect the cellular activity.22 However, comparing the moisture content with CDS and SIS scaffolds, the COL-F and COL/CS-F exhibited relatively lower moisture content; however, this did not affect the in vivo performance of these films, which demonstrated good tissue regeneration.25,413.2 Swelling ability
The percentage of swelling of COL-F and COL/CS-F samples at different time intervals is depicted in Fig. 1B. The COL-F sample showed slow absorption of water up to 6 h, with maximum water absorption of 119.7 ± 0.37%, beyond which the water uptake almost ceased. On the other hand, the COL/CS-F showed significantly higher swelling ability of 135.3 ± 0.471% (p < 0.0001) at the end of 1 h and was saturated at 12 h with percentage swelling of 175.8 ± 0.21%. It is also evident from the results of swelling behaviour that the amount of chitosan (1% w/v) used in this study is highly sufficient for producing hybrid film, thereby maintaining the inter and intra-molecular interactions between the collagen and chitosan molecules. Furthermore, the appreciable water holding capacity of the COL-F and COL/CS-F upon application will provide a high moisture level at the wound interface, which allows the absorption of wound exudates and permits the exchange of metabolic activities between the cell and scaffold.423.3 In vitro lysozyme degradation
The lysozyme degradation kinetics of the films are compared and presented in Fig. 1C. The degradation profile of the COL-F shows that there was significant weight loss of 40.8 ± 2.4% on day 21, compared to 24.3 ± 3.1% on day 7. A similar degradation pattern was also observed in COL/CS-F and accordingly, there was weight loss of 41.5 ± 0.3% on day 21 and 15.6 ± 1.1% on day 7. As a function of the increase in time, the biodegradation rate also increased in both films. However, on day 21, there was no significant difference in the COL-F and COL/CS-F degradation patterns (p > 0.05). A slow degrading ability of the wound healing dressing material is a good indication, due to the re-absorption of the matrix by the host. The degradation kinetics of COL-F and COL/CS-F show resistance to the rapid disintegration of the films. Moreover, the high degree of deacetylation (>85%) of chitosan used in this study could have avoided the rapid degradation of the chitosan, and eventually holds the collagen matrix tight. The balanced disassociation rate of the films enables neo-tissue formation at the wound interface, thereby enhancing tissue regeneration for longer periods. The presence of chitosan in COL-F makes it non-vulnerable to hydrolytic degradation and also helps in maintaining structural integrity for a prolonged duration.3.4 Water vapour transmission rate (WVTR)
The WVTR analysis is also an important physical parameter determination, which is usually carried out to assess the ability of the wound dressing material to retain absorbed water at the wound bed. It is highly essential for an ideal wound dressing material to control the water loss and to maintain a perceptible moist healing environment. Hence, an optimal WVTR is vital for favourable wound healing because high and low WVTR can cause integrity loss due to dryness and increase the risk of wound maceration and secondary infection.25 The WVTR values of COL-F and COL/CS-F were found to be 23.5 ± 0.6 g mm−2 h−1 and 29.1 ± 1.5 g mm−2 h−1, respectively, as reported in Fig. 1D. The significant increase in WVTR value (p < 0.05) observed for COL/CS-F indicates the increased transfer of absorbed water, due to the presence of chitosan within the film matrix. The WVTR values of COL-F and COL/CS-F were comparatively less than for other skin grafts.25,43 Such low WVTR values are more beneficial for dry wounds, where the wound exudate is considerably less. Accordingly, in this study, the created wound on the rat is relatively small (1.5 × 1.5 cm2), which on providing appropriate dressing material will dry faster and release significantly less wound exudates. There is early evidence of crest formation, also known as fibrinopurulent debris on day 4, as seen in Fig. 2. Therefore, during the initial days of wound healing (0–4) days, there is excessive wound exudate due to bleeding; however, once the crest is formed, there is considerable reduction in wound exudates. In such conditions, the influence of high WVTR can impede wound healing, due to over hydration and can cause tissue necrosis and bacterial infections. Therefore, COL-F and COL/CS-F can be used as occlusive dressings, thus catering to the wound bed with air-tight contact, thereby absorbing wound exudates and preventing fluid loss. | ||
| Fig. 2 Photographic representation of wound healing patterns in COL-F, COL/CS-F and control samples at regular time intervals. | ||
3.5 Microbial penetration test
The competence of the films to avoid microbial penetration and their growth was evaluated. Accordingly, the observed results from the optical density (OD) measurement show that both COL-F and COL/CS-F films would circumvent the microbial infiltration. There was no blurriness in the media and the OD values were statistically indifferent (p > 0.05) for COL-F and COL/CS-F, which were observed to be 0.006 ± 0.0005 and 0.007 ± 0.001, respectively. However, in the positive and negative controls, the OD values were 0.33 ± 0.024 and 2.01 ± 0.0241 (p < 0.05), respectively. The microbial penetration test shows that both COL-F and COL/CS-F were able to inhibit the penetration and infiltration of microbial growth. The presence of chitosan molecules within the COL-F also plays an additional role because chitosan itself is an active antimicrobial agent. Even though the underlying mechanism of chitosan being bacteriostatic remains unclear, the basic hypothesis relies on the intracellular leakages. The acidic nature of chitosan provides positive charges on the amino groups for the possible interaction with negative charges of cell membranes. This interaction is mediated by the electrostatic forces between the protonated NH3+ groups and negative residues, which cause the intracellular leakages and eventually lead to cell death.443.6 Wound morphology and period of epithelialisation
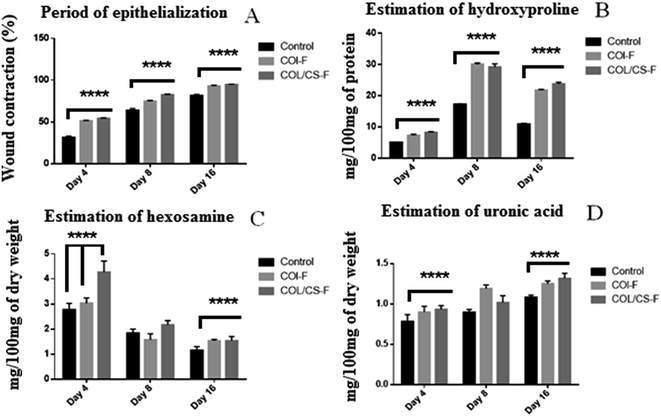
During the post-wounding period, no animal died and there was no presence of any adverse inflammatory reactions around the wounded area in all groups. Generally, the wound appeared to be red in colour on day 4, due to the formation of the crest, also known as fibrinopurulent debris. As the wound healing progressed, the crest was detachable in all groups and later appeared to be soft and smooth, due to tissue granulation as evident in the gross photographic illustration of the wound healing pattern (Fig. 2). The macroscopic evaluation of the wound healing progression is depicted in Fig. 3A. The rate of contraction increased in all the groups, but at different rates, with respect to the progression of time. The area of the wound contour decreased at much higher rates in the treated group, compared to that of the control animals. Amongst the treated groups, COL/CS-F group animals displayed faster healing, with 94.83 ± 0.46% wound closure on day 16, compared to 92.83 ± 0.95% in the chitosan-devoid COL-F treated group. The rate of wound healing in terms of wound contraction was observed to be COL/CS-F > COL-F > control. On comparing COL-F and COL/CS-F on day 16, there was no significant difference in their rates of epithelialization (p > 0.05). The enhanced rate of healing in both COL-F and COL/CS-F compared to the control groups can be attributed to the presence of native collagen in the films, which helps the fibroblast cells to initiate tissue granularization by producing ECM.6 It is also evident that the native collagen in the form of a film was able to coalesce with the wound bed and was readily acceptable by the host ECM. Similarly, the presence of chitosan in a fully-solubilised gel can synergise the bonding of free NH2 groups of chitosan with the functional groups at the wound bed, thereby coordinating the migration of chitosan molecules across the wound bed. Therefore, the migration of monomeric collagen and chitosan within the wound exudates can cause possible chemical bonding between the host ECM components such as fibronectin and GAGs.17,45 This bonding will ultimately lead to the biomimicking of the ECM by the films and eventually nourish the circulating cells in the wound environment for faster wound healing. In addition, the native collagen supplemented in film form can accelerate the migration of fibroblasts because the native collagen is “molecular” in nature and supplants endogenous collagen in vivo. This exchange of native and endogenous collagen can create active assimilation within wound tissue for better attachment, migration and proliferation of cells at the wound bed.46,47 The complete re-epithelization of COL-F and COL/CS-F was observed on day 16, whereas the CDS and SIS grafts demonstrated an early re-epithelization on day 14 of post wounding.25 Nevertheless, the re-epithelization rates of COL-F and COL/CS-F were in accordance with other biological materials reported in the literature, with excellent correlation favouring the planimetry and photographic results.3,6,493.7 Post wound skin constituents
The biochemical constituents of the re-epithelialized tissue were estimated in order to better comprehend the pattern of healing in different treatment groups. The biochemical parameters studied were collagen, hexosamine, uronic acid and soluble protein.| Non-wounded skin | Wounded skin | |
|---|---|---|
| Total protein expressed as mg/100 mg dry weight | ||
| Control | 0.923 ± 0.224 | 1.242 ± 0.037 |
| COL-F | 1.150 ± 0.040 | 1.380 ± 0.021 |
| COL/CS-F | 1.431 ± 0.037 | 1.562 ± 0.024 |
The estimation of biochemical parameters disclosed the evidence of early inflammation and rapid tissue granulation.
3.8 Status of glutathione and ascorbic acid
The concentrations of reduced glutathione and ascorbic acid in wounded and non-wounded areas of the same animal are presented in Table 2. High levels of reduced glutathione and ascorbic acid in both COL-F and COL/CS-F treated tissues were observed. When the individual groups were compared to the control group, the re-epithelialized skin tissue displayed an elevated level of the non-enzymatic antioxidants, compared to that of their normal skin tissue. Among the three different groups, the COL/CS-F treated wound tissue exhibited a high level of glutathione and ascorbic acid content, followed by the COL-F treated group and then the control groups. The respective unwounded specimens of these groups all followed the same trend. Usually, in the aftermath of tissue injury, the cytokine cascade is activated with the stimulation of phagocytic cells, which results in the formation of oxygen free-radicals and lipid peroxidation. Accordingly, COL-F and COL/CS-F possess good anti-oxidant properties with the ability to reverse the lipid peroxidative damages. It is reported that chitosan retains good antioxidant and scavenging properties, due to the higher degree of deacetylation, since it has more amino groups on the C2 glucan ring, which enhance the antioxidant properties.51 Accordingly, the chitosan used in this study is (>85%) deacetylated, which is highly sufficient for desirable oxidative stress scavenging, thereby avoiding delayed wound healing processes.| Antioxidants | Non-wounded skin | Wounded skin |
|---|---|---|
| Antioxidants are expressed as μg mg−1 protein | ||
| Glutathione | ||
| Control | 0.727 ± 0.012 | 0.983 ± 0.017 |
| COL-F | 1.150 ± 0.029 | 1.310 ± 0.055 |
| COL/CS-F | 1.347 ± 0.032 | 1.983 ± 0.073 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Ascorbic acid | ||
| Control | 0.733 ± 0.044 | 0.967 ± 0.033 |
| COL-F | 1.087 ± 0.041 | 1.407 ± 0.052 |
| COL/CS-F | 1.183 ± 0.044 | 1.633 ± 0.073 |
3.9 Histological observations
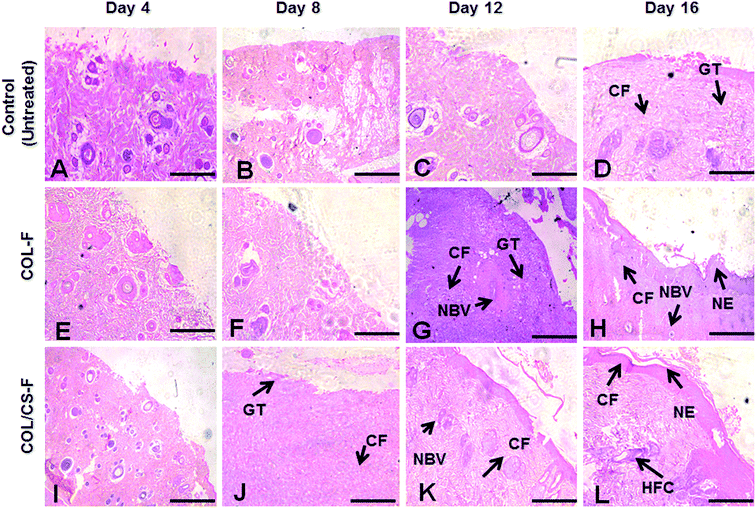
The H&E and MT staining of control tissue, COL-F and COL/CS-F are depicted in Fig. 4. During the early stages of wound healing, the inflammatory cells are being recruited at the wound interface for active haemostasis and phagocytosis. When compared to the control on day 16, the COL-F and COL/CS-F have more regular arrangements of collagen fibres with thick deposition of collagen bundles (Fig. 4H and L). This observation can be correlated with the wound contraction rate on day 16, which shows >90% of wound edges being pulled inwards, thereby reducing the defects due to effective epithelialization and tissue remodelling; whereas, in the control group on day 16, loosely packed and irregularly arranged collagen deposition was observed (Fig. 4D). Moreover, incomplete epithelialization with active fibroblasts and undifferentiated keratinocytes were seen beneath the basal lamina layer in the control groups.Similarly, the arrangement of newly formed collagen can be seen from the MT staining. On day 8, in all groups, the initiation of collagen remodelling at the wound bed can be seen from the presence of loose and reticulate collagen fibres. The newly formed collagen fibres appear to be disarrayed with a loose network orientation (Fig. 5B, F and J); whereas, on day 16, in the COL-F and COL/CS-F groups, the deposition of collagen bundles is well organised and maintained with consistent fibrillar aggregation (Fig. 5H and L). Compared to the experimental groups, on day 16 the control group (Fig. 5D) has a scattered collagen secretion with moderate collagen fibrous networking.
The histological observations seen in the epithelial tissue of COL-F and COL/CS-F would trigger cell-mediated, accelerated epidermal differentiation with organised collagen deposition in the absence of any immunogenic responses. A healing period of 16 days observed in the experimental groups shows the abrupt inflammatory response and maximal cell activation for migration and proliferation before 72 h. Therefore, the early inflammatory response to injury causes increased blood flow to provide optimal delivery of blood borne solutes for tissue repair.52–54 The presence of mature hair follicles, seen on day 16 on COL-F and COL/CS-F groups, is a good manifestation of epithelial cell migration. Therefore, the early formation of hair follicles would have expedited the acceleration of epithelium regeneration. Moreover, the normal architecture of the sebaceous glands, sudoriferous glands and well defined epidermis represents the appropriate applicability of the above films as cutaneous wound dressing material.6 Furthermore, the films being natural ECM derivatives, with intact native type I collagen can easily hasten the process of early wound healing by active integration with the host tissue for faster re-epithelialization and vascularisation.
3.10 NMR metabolic profiles
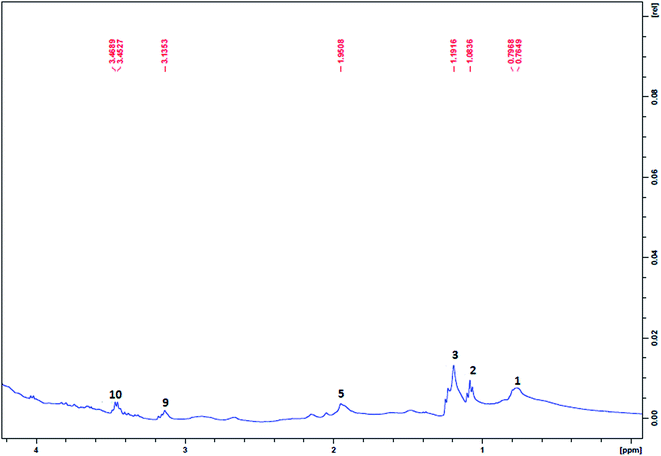
The NMR spectra of the plasma samples drawn at different post-wound time intervals from the control animals (untreated), the COL-F or COL/CS-F treated groups are provided in Fig. 6 and 7. The peaks of each spectrum correspond to the individual metabolites present in the circulating blood at periodic intervals over the course of healing, which is clearly depicted in Table 3. The metabolite profiles and their levels varied from one group to another at different healing time points. Therefore, these metabolites are considered as biological markers in the healing of the cutaneous wounds. A number of metabolites, including amino acids (alanine, leucine/isoleucine, glutamine and glycine), lipids and other energy metabolism related molecules (citrate, lactate, α-ketoglutarate, betaine, creatine and creatinine) have been identified in the plasma of wounded animals.55| Metabolite | Chemical shifts (1H ppm) | 4th day of plasma | 8th day of plasma | 12th day of plasma | 16th day of plasma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | COL-F | COL/CS-F | Control | COL-F | COL/CS-F | Control | COL-F | COL/CS-F | Control | COL-F | COL/CS-F | ||
| VLDL/LDL CH3 | 0.86 (br) | + | + | + | + | + | + | + | + | + | + | + | + |
| Isoleucine | 0.94 (t), 1.03 (d) | + | + | + | + | + | + | + | + | + | + | + | + |
| Leucine | 0.94 (t), 0.99 (t) | + | + | + | + | + | + | + | + | + | + | + | + |
| Lactate | 1.32 (d) | + | + | + | + | + | + | + | + | + | + | + | + |
| Alanine | 1.48 (d) | + | + | + | − | − | − | + | + | + | + | + | + |
| N-Acetyl glycoproteins | 2.04 (s) | + | + | + | + | + | + | + | + | + | + | + | + |
| Glutamine | 2.44 (m) | + | + | + | + | + | + | + | + | + | − | + | + |
| Citric acid | 2.94 (d) | + | + | + | + | + | + | + | + | + | + | + | + |
| α-Keto glutarate | 3.09 (s) | + | + | + | + | − | + | + | + | + | + | + | + |
| Betaine | 3.37 (m) | + | − | + | + | − | − | + | + | + | + | + | + |
| Glycine | 3.59 (m) | + | + | + | + | + | + | + | + | + | + | + | + |
| Creatine | 3.94 (m) | + | + | + | + | + | + | + | + | + | + | + | + |
| Creatinine | 3.04 (m), 4.05 (m) | + | + | + | + | + | + | + | + | + | + | + | + |
Several metabolites of the tricarboxylic acid cycle, creatinine cycle, and protein metabolism were identified in the NMR spectra of plasma samples of wounded animals. The 1H NMR spectra of the control samples, COL-F, COL/CS-F treatment groups, along with those of the plasma of unwounded (blank) animals were compared to distinguish the metabolic peaks responsible for the wound prognosis. The compositional differences in the films (COL-F or COL/CS-F) are also reflected in their performance on the wound surface, through variation in the kinetics of cellular activity. Aside from this, the variation in the biomaterial configuration also brought changes in the levels of certain metabolomes of the wounded animals. The plasma sample from control animals showed the presence of lipid metabolites, isoleucine & leucine, N-acetyl glycoproteins, betaine and glycine in small quantities (up to 8 days), as seen by smaller peaks in their respective absorption positions (Fig. 7A and B). In the case of treatment groups, the presence of isoleucine and leucine, in addition to lactate were noticed. Therefore, by comparing the unwounded (Fig. 6) with wounded samples, during the initial days of wound healing, apart from the presence of normal metabolites, the presence of creatine is invariably high in all three groups (control, COL-F and COL/CS-F). The formation of this metabolite through the creatinine biosynthetic pathway involves the reaction of arginine and glycine, which are known for their anti-inflammatory and anti-microbial properties;56 wound creation is always associated with inflammation and infection. The appearance of creatine only in the circulation of wounded animals is considered as an inflammatory response. The appearance of α-ketoglutarate in the animals of the experimental (COL-F and COL/CS-F) groups throughout the healing period refers to the enhanced utilization of glucose through the glycolysis linked TCA cycle. Moreover, the predominant presence of betaine helps to maintain the normal physiological functions of vital organs like the heart, liver, brain and kidneys.57 The elevated presence of betaine in COL-F and COL/CS-F treated groups suggests that this compound was over produced to maintain normal function in the skin as well. In the control group, betaine was detected in the first phase of healing (0–8 days); whereas, in the COL-F and COL/CS-F groups it was observed throughout the healing phase (8–16 days). Although the reason is unclear, it is speculated that the healing in the treated group was taken care of by the topically applied films in the initial stage and therefore, it did not require an endogenous metabolite for normalization of the physiological function, and its appearance at the later time may be correlated to its necessity in the proliferation and remodelling. This metabolomic peak (betaine) suggests that (i) the wound creation triggers a creatinine biosynthetic pathway; (ii) COL-F or COL/CS-F treatment of wounds causes the production of α ketoglutarate through the TCA cycle in the early phase of healing; (iii) in the case of the control group, betaine was produced in the early phase; (iv) the appearance of the amino acids isoleucine and leucine during 8–16 days (Fig. 7C and D) is attributed to the remodelling of the wound where the cleavage of proteins takes place by matrix metalloproteinases (MMPs) and their cleavage products are released in the circulation;58 (v) the presence of lactate during 8–12 days features the absence of the availability of pyruvate to the TCA cycle at the healing site. From these results, it appears that amino acids (arginine and glycine) are utilized in the control group in the early stage, whereas in the COL-F and COL/CS-F groups, glucose is utilized in the early stage (inflammation and proliferation) and amino acids (leucine and isoleucine) are released in the later stage.
This in vivo study demonstrated that both these films show excellent prognosis in wound healing, due to their individual macro-structural architectures, which play a vital role in cell attachment and proliferation. The excellent physical and biological properties of COL-F and COL/CS-F have catered to the need to use these films as wound dressing material. However, the COL/CS-F showed no significant difference in terms of re-epithelization rate, compared to COL-F; nevertheless, we observed a possible enhanced healing rate when the two biomaterials (collagen and chitosan) were applied together. Therefore, we consider that the presence of chitosan in the COL-F accelerated wound healing by exerting relevant biomimetic and chemotactic effects at the wound surface. Thus, this study proved the intrinsic efficiency of these films in treating and promoting wound healing through promising and functional pre-clinical outcomes.
4. Conclusion
Herein, we have investigated the potential healing ability of COL-F and COL/CS-F films in treating cutaneous wounds. The treatment of COL-F with chitosan improved its overall characteristics through its additional amino groups, which act as a binding site with collagen to enhance the overall biological properties and stability. The results of our study suggest that the developed COL/CS-F could be a better functional biomaterial for targeted clinical applications, although COL-F also performs well. NMR based technology used for the metabolomics analysis of cutaneous wounds in rats highlights the changed pattern of metabolic pathways during the process of healing. The important pathways that were found to be altered are glucose metabolism, TCA cycle and amino acid metabolism. The metabolites described in this study may be used as markers to understand the status of wound healing. In conclusion, the results of this study have shown the potential wound healing efficacy of COL-F or COL/CS-F, and the corresponding metabolomics profile provided valuable biochemical insight into the metabolic disturbances in wounded conditions.Conflict of interest
The authors declare there is no conflict of interest regarding the publication of this paper.Acknowledgements
This work is supported by the Ministry of Environment and Forests (Grant No. F. No. 19-28/2007-RE, dated 23-01-2012), Government of India, New Delhi. Author(s) acknowledge NMR facility support from STRAIT (CSC 0201) under the XII five-year plan project of CSIR-CLRI.References
- R. Guo, J. Teng, S. Xu, L. Ma, A. Huang and C. Gao, Wound Repair Regeneration, 2014, 22(3), 390–398 CrossRef PubMed.
- A. Vasconcelos, A. C. Gomes and A. Cavaco-Paulo, Novel silk fibroin/elastin wound dressings, Acta Biomater., 2012, 8(8), 3049–3060 CrossRef CAS PubMed.
- T. Muthukumar, D. Prakash, K. Anbarasu, B. S. Kumar and T. P. Sastry, RSC Adv., 2014, 4, 64267–64276 RSC.
- S. Thirupathi Kumara Raja, T. Thiruselvi, R. Aravindhan, A. B. Mandal and A. Gnanamani, J. Mater. Chem. B, 2015, 3, 1230–1244 RSC.
- T. V. Anilkumar, J. Muhamed, A. Jose, A. Jyothi, P. V. Mohanan and L. K. Krishnan, Biologicals, 2011, 39(2), 81–88 CrossRef CAS PubMed.
- R. Judith, M. Nithya, C. Rose and A. B. Mandal, Life Sciences, 2010, 87(1–2), 1–8 CrossRef CAS PubMed.
- D. Wainwright, M. Madden, A. Luterman, J. Hunt, W. Monafo and D. Heimbach, et al., J. Burn Care Rehabil., 1996, 17, 124–136 CrossRef CAS PubMed.
- D. J. Wainwright, Burns, 1995, 21, 243–248 CrossRef CAS PubMed.
- X. Wang, C. You, X. Hu, Y. Zheng, Q. Li, Z. Feng, H. Sun, C. Gao and C. Han, Acta Biomater., 2013, 9(8), 7822–7832 CrossRef CAS PubMed.
- W. Dai, N. Kawazoe, X. Lin, J. Dong and G. Chen, Biomaterials, 2010, 31, 2141–2152 CrossRef CAS PubMed.
- G. Chen, T. Ushida and T. Tateishi, Macromol. Biosci., 2002, 2, 67–77 CrossRef CAS.
- X. Chen, Y. Y. Qi, L. L. Wang, Z. Yin, G. L. Yin and X. H. Zou, Biomaterials, 2008, 29, 3683–3692 CrossRef CAS PubMed.
- L. Ma, C. Gao, Z. Mao, J. Zhou, J. Shen, X. Hu and C. Han, Biomaterials, 2003, 24(26), 4833–4841 CrossRef CAS PubMed.
- P. Jithendra, A. M. Rajam, T. Kalaivani, A. B. Mandal and C. Rose, ACS Appl. Mater. Interfaces, 2013, 5(15), 7291–7298 CAS.
- P. He, S. S. Davis and L. Illum, Int. J. Pharm., 1998, 166(1), 75–88 CrossRef CAS.
- P. Calvo, S. S. Vila-Jato and M. J. Alonso, Int. J. Pharm., 1997, 153(1), 41–50 CrossRef CAS.
- M. Nithya, L. Suguna and C. Rose, Biochemica et Biophysica Acta, 2003, 1620(1–3), 25–31 CrossRef CAS.
- M. Rajam, S. Pulavendran, C. Rose and A. B. Mandal, Int. J. Pharm., 2011, 410(1–2), 145–152 CrossRef CAS PubMed.
- J. Lin, C. Li, Y. Zhao, J. Hu and L. M. Zhang, ACS Appl. Mater. Interfaces, 2012, 4(2), 1050–1057 CAS.
- S. S. Silva, S. G. Caridade, J. F. Mano and R. L. Reis, Carbohydr. Polym., 2013, 98(1), 581–588 CrossRef CAS PubMed.
- K. W. Ng and D. W. Hutmacher, Biomaterials, 2006, 27(26), 4591–4598 CrossRef CAS PubMed.
- S. F. Badylak, D. O. Freytes and T. W. Gilbert, Acta Biomater., 2009, 5(1), 1–13 CrossRef CAS PubMed.
- C. A. Barnes, J. Brison, R. Michel, B. N. Brown, D. G. Castner, S. F. Badylak and B. D. Ratner, Biomaterials, 2011, 32(1), 137–143 CrossRef CAS PubMed.
- M. S. Kim, K. D. Hong and H. W. Shin, Int. J. Biol. Macromol., 2005, 36, 54–60 CrossRef CAS PubMed.
- D. Revi, V. Prabhakaran Vineetha, J. Muhamed, A. Rajan and T. V. Anilkumar, J. Tissue Eng., 2013, 4, 2041731413518060 Search PubMed.
- K. Gopal Shankar, S. Udhaya Kumar, S. Sowndarya, P. Suresh Babu and C. Rose, J. Biomater. Appl., 2016, 30(6), 780–792 CrossRef CAS PubMed.
- M. Pandima Devi, T. P. Sastry and S. Meignanalakshmi, J. Pharm., 2012, 2(6), 21–32 Search PubMed.
- C. Tangsadthakun, S. Kanokpanont, N. Sanchavanakit, R. Pichyangkura, T. Banaprasert, Y. Tabata and S. Damrongsakkul, J. Biomater. Sci., Polym. Ed., 2007, 18(2), 147–163 CrossRef CAS PubMed.
- P. W. Morgen, A. G. Binnington, C. W. Miller, D. A. Smith, A. Valliant and J. F. Prescott, Veterinary Surgery, 1994, 23, 494–502 CrossRef.
- J. F. Woessner, Arch. Biochem. Biophys., 1961, 93(2), 440–447 CrossRef CAS PubMed.
- L. A. Elson and W. T. J. Morgan, Biochem. J., 1933, 27, 1824–1828 CrossRef CAS PubMed.
- T. Bitter and H. M. Muir, Anal. Biochem., 1962, 4, 330–334 CrossRef CAS PubMed.
- S. Schiller, A. Slover and A. Dorfman, J. Biol. Chem., 1961, 236(2), 983–987 CAS.
- O. H. Lowry, N. J. Resenbrough, A. L. Farr and R. J. Randal, J. Biol. Chem., 1951, 193(1), 265–275 CAS.
- M. S. Moron, Biochemica Biophysica Acta, 1979, 582(1), 67–74 CrossRef CAS.
- S. T. Omaye, J. D. Turnball and H. E. Sauberlich, Methods Enzymol., 1971, 62(1), 3–11 Search PubMed.
- D. Sheehan and B. Hrapchak, Theory and practice of histotechnology, Battelle Press, Columbus, OH, 2nd edn, 1980, pp. 189–190 Search PubMed.
- D. R. Knighton, T. K. Hunt, K. K. Thakral and W. H. Goodson, Ann. Surg., 1982, 196, 379–388 CrossRef CAS.
- E. T. Dominguez, F. Castro-Munozledo and W. Kuri-Harcuch, Cell Tissue Res., 2002, 307(1), 79–89 CrossRef PubMed.
- R. Tsuboi and D. B. Rifkin, J. Exp. Med., 1990, 172, 245–251 CrossRef CAS PubMed.
- J. Bryan, Journal of Wound Care, 2004, 13, 227–228 CrossRef CAS PubMed.
- M. Szycher and S. J. Lee, J. Biomater. Appl., 1992, 7, 142–213 CrossRef CAS PubMed.
- A. Mittal and N. Kumar, Pharm. Res., 2012, 29(11), 3110–3121 CrossRef CAS PubMed.
- S. J. Jeon, M. Oh, W.-S. Yeo, K. N. Galvão and K. C. Jeong, PLoS ONE, 2014, 9(3), e92723, DOI:10.1371/journal.pone.0092723.
- C. Rose and A. B. Mandal, Int. J. Biol. Macromol., 1996, 18(1–2), 41–53 CrossRef CAS PubMed.
- C. Rose, M. Kumar and A. B. Mandal, Biochem. J., 1988, 249(1), 127–133 CrossRef CAS PubMed.
- P. Martin, J. Hopkinson-woodley and J. McCluskey, Prog. Growth Factor Res., 1992, 4(2), 25–44 CrossRef CAS PubMed.
- B. K. Pilcher, J. A. Dumin, B. D. Sudbeck, S. M. Krane, H. G. Welgus and W. C. Parks, J. Cell Biol., 1997, 137, 1445–1457 CrossRef CAS PubMed.
- S. Singaravelu, G. Ramanathan, T. Muthukumar, D. Raja, N. Nagiah, S. Thyagarajan, A. Adithan, P. Gunasekaran, T. S. Natarajan, G. V. N. Geetha Selva, K. Jong-Hoon and U. Tirichurapalli Sivagnanam, J. Mater. Chem. B, 2016, 4, 3982–3997 RSC.
- S. E. Noorjahan and T. P. Sastry, J. Biomed. Mater. Res., Part B, 2004, 71(2), 305–312 CrossRef CAS PubMed.
- M.-T. Yen, J.-H. Yang and J.-L. Mau, Carbohydr. Polym., 2012, 74, 840–844 CrossRef.
- T. J. Koh and L. A. DiPietro, Expert Rev. Mol. Med., 2011, 13, e23, DOI:10.1017/S1462399411001943.
- J. D. Stroncek and W. M. Reichert, Overview of Wound Healing in Different Tissue Types, CRC Press/Taylor & Francis, Boca Raton (FL), USA, 2008, ch. 1, pp. 1–64 Search PubMed.
- Y. M. Fei, J. Zainol, A. G. Pillay and N. Yusof, J. Med. Sci., 2002, 2, 1–6 CrossRef.
- L. Jiang, X. Zhao, C. Huang, H. Lei, H. Tang and Y. Wang, Mol. BioSyst., 2014, 10, 2914–2922 RSC.
- P. Li, Y.-L. Yin, D. Li, S. Woo Kim and G. Wu, Br. J. Nutr., 2007, 98, 237–252 CrossRef CAS PubMed.
- V. B. Patel and K. Mehta, Betaine in context, Published by the Royal Society of Chemistry, London, 2015, ch. 1, pp. 3–8 Search PubMed.
- H. F. Bigg, A. D. Rowan, M. D. Barker and T. E. Cawston, FEBS J., 2007, 274(5), 1246–1255 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |