pH-sensitive drug release of star-shaped micelles with OEG brush corona†
Abstract
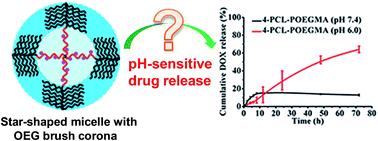
Star-shaped polymers are attractive as drug carriers due to the integration of relatively easy synthesis compared to the preparation of hyper-branched dendrimers and their potential ability to form unimolecular nanoparticles with enhanced stability to survive the high dilution-associated deformation and subsequently premature drug release in the body fluids and blood during circulation. Clearly, the star structure exerts a significant effect on their bioproperties and potential applications. In this study, three star-shaped amphiphilic copolymers with the same polymer compositions but different star structures were synthesized to investigate the effect of star architecture on their properties as well as potential applications as drug carriers. Three different branched alcohols, 2-(hydroxymethyl) propane-1,3-diol, pentaerythritol and dipentaerythritol with 3, 4, and 6 respective hydroxyl groups were chosen as the starting core to generate the target star-shaped amphiphilic block copolymers composed of hydrophobic poly(ε-caprolactone) (PCL) and hydrophilic poly(oligoethyleneglycol methacrylate) (POEGMA) with 3, 4, and 6 corresponding star arms by a combination of ring-opening polymerization (ROP) and atom transfer radical polymerization (ATRP). Dynamic light scattering (DLS) measurements and pyrene fluorescence probe technique confirmed the capability of the resultant star-shaped amphiphilic copolymers to form unimolecular micelles with average diameters smaller than 50 nm in an aqueous phase. A comparison of the drug loading capacity revealed that the micelles of 4-star-PCL–POEGMA (4s-PCL–POEGMA) exhibited the highest drug loading content (DLC) of all the three formulations. More importantly, in vitro doxorubicin (DOX) release study showed unique pH-mediated drug release behaviors, i.e., dramatically accelerated release at pH 6.0 but a much slower profile at pH 7.4, from these generally recognized “pH-insensitive” 4-arm star-shaped micelles. An acid-triggered degradation and an acid–base titration were performed to further reveal that such interesting pH-responsive drug release behaviors were attributed primarily to the hydrophilic corona of OEG brushes. This study is believed to provide a new insight into the structure–bioproperties relationship of star-shaped polymers, thus should be useful for the future design and development of novel star-shaped polymers for controlled drug delivery.

 Please wait while we load your content...
Please wait while we load your content...