A unique 3D ultramicroporous triptycene-based polyimide framework for efficient gas sorption applications†
Abstract
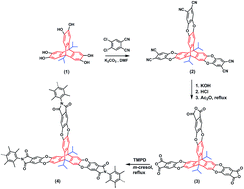
A novel 3D ultramicroporous triptycene-based polyimide framework with high surface area (1050 m2 g−1) and thermal stability was synthesized. It exhibits relatively high CO2 (3.4 mmol g−1 at 273 K and 1 bar), H2 (7 mmol g−1 at 77 K and 1 bar), and olefin sorption capacity, good CO2/N2 (45) and CO2/CH4 (9.6) selectivity at 273 K and 1 bar, as well as promising C2H4/CH4 and C3H6/CH4 selectivities at 298 K, making it a potential candidate for CO2 capture, H2 storage, and hydrocarbon gas separation applications.

 Please wait while we load your content...
Please wait while we load your content...