Sequential hydroarylation/Prins cyclization: an efficient strategy for the synthesis of angularly fused tetrahydro-2H-pyrano[3,4-c]quinolines†
M. Rajashekhar Reddya,
G. Ravi Kumara,
Suresh Yarlagaddaa,
C. Ravikumar Reddya,
J. S. Yadava,
B. Sridharb and
B. V. Subba Reddy*a
aCentre for Semiochemicals, CSIR-Indian Institute of Chemical Technology, Hyderabad, India. E-mail: basireddy@iict.res.in; Web: http://www.iictindia.org Fax: +91-40-27160512
bLaboratory of X-ray Crystallography, CSIR-Indian Institute of Chemical Technology, Hyderabad, India
First published on 28th November 2016
Abstract
Various aldehydes undergo a smooth cascade cyclization with N-(5-hydroxypent-2-yn-1-yl)-4-methyl-N-phenylbenzenesulfonamide in the presence of 10 mol% Ph3PAuCl/AgSbF6/In(OTf)3 to furnish the corresponding tetrahydro-2H-pyrano[3,4-c]quinoline derivatives in good yields with high selectivity. This is the first report on the synthesis of tetrahydro-2H-pyrano[3,4-c]quinolines through a tandem hydroarylation/Prins cyclization process.
Introduction
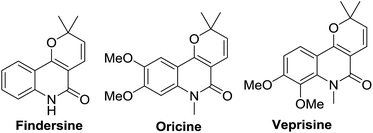
Nitrogen-containing heterocycles are common motifs in natural and synthetic molecules of pharmaceutical and agrochemical significance.1 Among them, quinolin-2(1H)-one and its derivatives are ubiquitous subunits of numerous natural products, as well as being an extremely important class of heterocycles due to their wide range of applications in medicinal chemistry.2–5 In particular, the pyranoquinoline skeleton is often found in various natural products (Fig. 1).6 These alkaloids and their derivatives possess a wide range of pharmacological activities such as anti-allergic, anti-inflammatory, anti-pyretic, psychotropic and anti-platelet properties.7,8 Thus, the synthesis of pyranoquinoline derivatives is, currently, of great interest.Recently, there is a remarkable advancement in Prins cyclization for the stereoselective synthesis of oxygen containing heterocycles.9 In particular, its intramolecular variation provides a large number of fused/bridged heterocycles and spirocycles such as bicyclo[3.3.1]nonanes, bicyclo[3,2,1]octanes, and oxaspirobicycles.10 Therefore, it has become a powerful tool for the stereoselective construction of fused-, bridged- and spiro-skeletons. In spite of its potential application in natural products synthesis,11 the scope of this tandem approach has not yet been explored to produce angularly fused tetrahydro-2H-pyrano[3,4-c]quinolines from readily accessible aldehydes and aryl tethered alkynols. Recently, metal catalyzed hydroarylation of alkynes has gained importance in the synthesis of biologically important heterocycles.12 Among them, Au-catalyzed hydroarylation of alkynes has attained utmost popularity due to their high alkynophilicity and Lewis acidity to promote further C–C or C–X bond formation.13,14
Results and discussions
Inspired by a recent advancement in multi-catalyst system,15 we herein disclose a novel cascade strategy for the one-pot synthesis of tetrahydro-2H-pyrano[3,4-c]quinoline derivatives from N-(5-hydroxypent-2-yn-1-yl)-4-methyl-N-phenylbenzene sulfonamide and aldehydes through a multi-catalytic cascade process. As a model reaction, we attempted the coupling of 4a with p-anisaldehyde (5a) using different catalysts. Accordingly, we performed a systematic screening of catalysts in the above reaction (Table 1). To our surprise, no desired product (6a) was obtained by using various Lewis acids such as InCl3, Sc(OTf)3, BF3·OEt2, TMSOTf and AgSbF6 (entries a–e, Table 1). However, the use of 10 mol% In(OTf)3 gave the expected product 6a in low to moderate yields (entries f and g, Table 1). These initial findings prompted us to find out an effective catalytic system for this reaction. Therefore, we further performed this reaction using the combination of In(OTf)3 and AgSbF6. But there was no significant improvement in yield (entry h, Table 1). Furthermore, the use of 10 mol% PPh3AuCl also didn't give the desired product (entry i, Table 1). Though the combination of PPh3AuCl/AgSbF6 facilitates the initial hydroarylation, it fails to give the desired product (entry j, Table 1). After screening several catalysts, 10 mol% Ph3PAuCl/AgSbF6/In(OTf)3 in DCE at 0 °C was found to be the best optimized conditions to achieve the product in excellent yield (entry k, Table 1).| Entry | Lewis acid | Equiv. | Solvent | Temp. (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Yield refers to pure products after column chromatography. | ||||||
| a | lnCl3 | 0.1 | DCM | rt | 12 | — |
| b | Sc(OTf)3 | 0.1 | DCM | rt | 12 | — |
| c | BF3·OEt2 | 1.0 | DCM | rt | 12 | — |
| d | TMSOTf | 1.0 | DCM | rt | 12 | — |
| e | AgSbF6 | 0.1 | DCM | rt | 12 | — |
| f | ln(OTf)3 | 0.1 | DCM | rt | 12 | 10 |
| g | ln(OTf)3 | 0.1 | DCE | 70 | 2 | 60 |
| h | ln(OTf)3/AgSbF6 | 0.1 | DCE | rt | 2 | 62 |
| i | PPh3AuCl | 0.1 | DCM | rt | 12 | — |
| j | PPh3AuCl/AgSbF6 | 0.1 | DCM | rt | 12 | — |
| k | PPh3AuCl/AgSbF6/ln(OTf)3 | 0.1 | DCE | 0 | 2 | 88 |
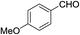
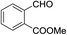
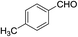
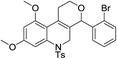
In the above catalytic system, Au(I) activates the alkyne moiety to facilitate the hydroarylation and AgSbF6 is expected to accelerate the reaction by generating active Au(I) cationic species. In(OTf)3 acts as a Lewis acid and hence it activates the aldehyde effectively. Therefore, the cooperative effect of these catalysts facilitates this cascade process. As shown in Table 1, the combination of 10 mol% of each catalyst gave the product 6a in 88% after 2.0 h in dichloroethane at 0 °C (entry k, Table 1). No further increase in yield of 6a was observed by elevating the temperature to 25 °C. After successful standardization of reaction conditions, we extended this method to other aldehydes. Interestingly, different aldehydes including aromatic and aliphatic participated well in this cascade process. As shown in Table 2, the substituent present on the aromatic ring showed some effect on the conversion. Indeed, electron rich aldehydes such as p-methoxy-, p-methyl- and p-ethyl benzaldehydes gave the product relatively in higher yields (entries a, g, and l, Table 2) than electron deficient substrates (entries c, e, and f, Table 2). Among halogenated aromatic aldehydes, p- or o-bromobenzaldehyde afforded the product relatively in higher yield than fluoro- or chloro-substituted aromatic aldehyde (entries b, o and h Table 2). Among heterocyclic aldehydes, thiophene-2-carboxaldehyde gave the product relatively in good yield (entry i, Table 2). However, aliphatic aldehydes such as n-propanal and isobutyraldehyde furnished the products comparatively in lower yields (entries j, and k, Table 2) than aromatic and heterocyclic substrates. Furthermore, sterically hindered substrates, for example, 1-naphthaldehyde and 2-bromobenzaldehyde (entries m and n, Table 2) participated well in this reaction. The scope of the reaction was further illustrated with respect to ketones such as α-tetralone and N-methylisatin. However, these cyclic ketones failed to undergo this domino cyclization (entries p and q, Table 2). The reaction was performed with different N-protecting groups such as N-benzyl and N-mesyl and with unprotected NH. To our surprise, no desired product was formed when the reaction was conducted with substrates bearing N-benzyl and NH groups (entry t, Table 2). Low yield (20%) was achieved with N-mesyl group (entry r, Table 2). The reaction was quite successful only with N-Ts group (Table 2). This method is further extended to other substrates like N-(5-hydroxypent-2-yn-1-yl)-4-methyl-N-phenylbenzenesulfonamide and 5-(3,4,5-trimethoxyphenoxy)pent-3-yn-1-ol. The reaction between N-(5-hydroxypent-2-yn-1-yl)-4-methyl-N-phenylbenzenesulfonamide and p-fluorobenzaldehyde failed to give the desired product (entry s, Table 2). Similarly, the reaction of 5-(3,4,5-trimethoxyphenoxy)pent-3-yn-1-ol with pyridine-2-carboxaldehyde also didn't give the expected product under the present reaction conditions. The structure of 6j was confirmed by a single crystal X-ray diffraction (Fig. 2).16
The reaction was proposed to be proceeded through the formation of Au–π complex (A) by a coordination of cationic Au(I) species with alkyne moiety. A subsequent attack of the aryl group affords the cyclic vinylidene–Au complex (B). Concurrently, the pendent alcohol reacts with aldehyde activated likely by In(III) to afford the E-oxocarbenium ion (C), which is trapped intramolecularly by a gold vinylidene species to produce the tetrahydropyranoquinoline (6) with a regeneration of the gold catalyst (Scheme 1).
On the other hand, In(OTf)3 alone can activate the alkyne under heating conditions to facilitate the cyclization.17
Conclusion
In summary, a novel cascade strategy has been developed for the synthesis of tetrahydro-2H-pyrano[3,4-c]quinoline derivatives using a trifunctional catalytic system. This method facilitates the sequential formation of C–C, C–O, and C–C bonds with a wide substrate scope under relatively mild and neutral conditions, which makes it an attractive strategy. This approach demonstrates the synergism between gold, silver and indium complexes for domino cyclization.Experimental section
General
All the solvents were dried according to standard literature procedures. Reactions were performed in oven-dried round bottom flask, the flasks were fitted with rubber septa and reactions were conducted under nitrogen atmosphere. Glass syringes were used to transfer solvent. Crude products were purified by column chromatography on silica gel of 60–120 or 100–200 mesh. Thin layer chromatography plates were visualized by exposure to ultraviolet light and/or by exposure to iodine vapors and/or by exposure to methanolic acidic solution of p-anisaldehyde followed by heating (<1 min) on a hot plate (∼250 °C). Organic solutions were concentrated on rotary evaporator at 35–40 °C. IR spectra were recorded on FT-IR spectrometer. 1H NMR and 13C NMR (proton-decoupled) spectra were recorded in CDCl3 solvent on 200, 300, 400 or 500 MHz NMR spectrometer. Chemical shifts (δ) were reported in parts per million (ppm) with respect to TMS as an internal standard. Coupling constants (J) are quoted in hertz (Hz). Mass spectra were recorded on mass spectrometer by electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) technique.Acknowledgements
GRK, SY and MRR, CRR, thank UGC and CSIR respectively for the award of fellowships. BVS thanks CSIR, New Delhi for financial support as part of XII Five Year plan under title ORIGIN (CSC-0108).Notes and references
- For selected reviews, see:
(a) L. Yet, Chem. Rev., 2000, 100, 2963 CrossRef CAS PubMed
; (b) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed
; (c) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed
; (d) J. Jiricek and S. Blechert, J. Am. Chem. Soc., 2004, 126, 3534 CrossRef CAS PubMed
.
-
(a) P. Hewawasam, W. Fan, M. Ding, K. Flint, D. Cook, G. D. Goggings, R. A. Myers, V. K. Gribkoff, C. G. Boissard, S. I. Dworetzky, J. E. Starret and N. Lodge, J. Med. Chem., 2003, 46, 2819 CrossRef CAS PubMed
; (b) B. M. Andresen, M. Couturier, B. Cronin, M. D'Occhio, M. D. Ewing, M. Guinn, J. M. Hawkins, V. J. Jasys, S. D. LaGreca, J. P. Lyssikatos, G. Moraski, K. Ng, J. W. Raggon, A. M. Stewart, D. L. Tickner, J. L. Tucker, F. J. Urban, E. Vazquez and L. Wei, Org. Process Res. Dev., 2004, 8, 643 CrossRef CAS
; (c) P. Hewawasam, W. Fan, J. Knipe, S. L. Moon, C. G. Boissard, V. K. Gribkoff and J. E. Starret, Bioorg. Med. Chem. Lett., 2002, 12, 1779 CrossRef CAS PubMed
; (d) Y.-Q. Fang, R. Karisch and M. Lautens, J. Org. Chem., 2007, 72, 1341 CrossRef CAS PubMed
; (e) A. Wong, J. T. Kuethe, I. W. Davies and D. L. Hughes, J. Org. Chem., 2004, 69, 7761 CrossRef CAS PubMed
; (f) N. Ribeiro, H. Tabaka, J. Peluso, L. Fetzer, C. Nebigil, S. Dumont, C. D. Muller and L. Desaubry, Bioorg. Med. Chem. Lett., 2007, 17, 5523 CrossRef CAS PubMed
.
-
(a) K. M. Witherup, R. W. Ransom, A. C. Graham, A. M. Bernard, M. J. Salvatore, W. C. Lumma, P. S. Anderson, S. M. Pitzenberger and S. L. Varga, J. Am. Chem. Soc., 1995, 117, 6682 CrossRef CAS
; (b) F. Zhou, J. Liu, K. Ding, J. Liu and Q. Cai, J. Org. Chem., 2011, 76, 5346 CrossRef CAS PubMed
; (c) D. Ma, C. Xia, J. Jiang and J. Zhang, Org. Lett., 2001, 3, 2189 CrossRef CAS PubMed
.
-
(a) Y. Sangoi, O. Sakuleko, S. Yuenyongsawad, A. Kanjana-opas, K. Ingkaninan, A. Plubrukan and K. Suwanborirux, Mar. Drugs, 2008, 6, 578 CrossRef
; (b) P. W. Okanya, K. I. Mohr, K. Gerth, R. Jansen and R. J. Muller, J. Nat. Prod., 2011, 74, 603 CrossRef CAS PubMed
; (c) L. Ni, Z. Li, F. Wu, J. Xu, X. Wu, L. Kong and H. Yao, Tetrahedron Lett., 2012, 53, 1271 CrossRef CAS
; (d) A. J. Carroll, S. Duffy and V. M. Avery, J. Org. Chem., 2010, 75, 8291 CrossRef CAS PubMed
; (e) D. J. Panarese and C. W. Lindsley, Org. Lett., 2012, 14, 5808 CrossRef PubMed
.
-
(a) F. Pierre, P. C. Chua, E. O'Brien, A. Siddiqui-Jain, P. Bourbon, M. Haddach, J. Michaux, J. Nagasawa, M. K. Schwaebe, E. Stefan, A. Vialettes, J. P. Whitten, T. K. Chen, L. Darjania, R. Stansfield, K. Anderes, J. Bliesath, D. Drygin, C. Ho, M. Omori, C. Proffitt, N. Streiner, K. Trent, W. G. Rice and D. M. Ryckman, J. Med. Chem., 2011, 54, 635 CrossRef CAS PubMed
; (b) J. Dogan Koruznjak, M. Grdisa, N. Slade, B. Zamola, K. Pavelic and G. Karminski-Zamola, J. Med. Chem., 2003, 45, 4516 CrossRef PubMed
; (c) M. Aleksic, B. Bertosa, R. Nhili, L. Uzelac, I. Jarak, S. Depauw, M.-H. David-Cordonnier, M. Kralj, S. Tomic and G. Karminski-Zamola, J. Med. Chem., 2012, 55, 5044 CrossRef CAS PubMed
.
-
(a) M. Ramesh, P. S. Mohan and P. Shanmugam, Tetrahedron, 1984, 40, 4014 Search PubMed
; (b) M. F. Grundon, The Alkaloids: Quinoline Alkaloids Related to Anthranilic Acid, Academic Press, London, 1988, vol. 32, p. 341 Search PubMed
.
-
(a) N. Yamada, S. Kadowaki, K. Takahashi and K. Umezu, Biochem. Pharmacol., 1992, 44, 1211 CrossRef CAS PubMed
; (b) K. Faber, H. Stueckler and T. Kappe, Heterocycl. Chem., 1984, 21, 1177 CrossRef CAS
; (c) J. V. Johnson, S. Rauckman, P. D. Baccanari and B. Roth, J. Med. Chem., 1989, 32, 1942 CrossRef CAS PubMed
.
-
(a) D. Mabire, S. Coupa, C. Adelinet, A. Poncelet, Y. Simonnet, M. Venet, R. Wouters, A. S. J. Lesage, L. V. Beijsterveldt and F. Bischoff, J. Med. Chem., 2005, 48, 2134 CrossRef CAS PubMed
; (b) J. P. Michael, Nat. Prod. Rep., 2002, 19, 742 RSC
; (c) J. P. Michael, Nat. Prod. Rep., 2003, 20, 476 RSC
; (d) J. P. Michael, Nat. Prod. Rep., 2004, 21, 650 RSC
; (e) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC
.
-
(a) C. Olier, M. Kaafarani, S. S. Gastaldi and M. P. Bertrand, Tetrahedron, 2010, 66, 413 CrossRef CAS
; (b) I. M. Pastor and M. Yus, Curr. Org. Chem., 2007, 11, 925 CrossRef CAS
; (c) M. Pastor and I. M. Yus, Curr. Org. Chem., 2012, 16, 1277 CrossRef
; (d) O. L. Epstein and T. Rovis, J. Am. Chem. Soc., 2006, 128, 16480 CrossRef CAS PubMed
; (e) Y. Kishi, H. Nagura, S. Inagi and T. Fuchigami, Chem. Commun., 2008, 3876 RSC
; (f) K. Zheng, X. Liu, S. Qin, M. Xie, L. Lin, C. Hu and X. J. Feng, J. Am. Chem. Soc., 2012, 134, 17564 CrossRef CAS PubMed
; (g) A. Diez-Varga, H. Barbero, F. J. Pulido, A. Gonzalez-Ortega and A. Barbero, Chem.–Eur. J., 2014, 20, 14112 CrossRef CAS PubMed
.
-
(a) Z.-H. Chen, Y.-Q. Tu, S.-Y. Zhang and F.-M. Zhang, Org. Lett., 2011, 13, 724 CrossRef CAS PubMed
; (b) S. N. Chavre, P. R. Ullapu, S.-J. Min, J. K. Lee, A. N. Pae, Y. Kim and Y. S. Cho, Org. Lett., 2009, 11, 3834 CrossRef CAS PubMed
; (c) J. D. Elsworth and C. L. Willis, Chem. Commun., 2008, 1587 RSC
; (d) A. Barbero, A. Diez-Varga and F. J. Pulido, Org. Lett., 2013, 15, 5234 CrossRef CAS PubMed
; (e) K. P. Minor and L. E. Overman, Tetrahedron, 1997, 53, 8927 CrossRef CAS
; (f) B. V. S. Reddy, V. Swathi, S. Manisha, M. P. Bhadra, B. Sridhar, D. Satyanarayana and B. Jagadeesh, Org. Lett., 2014, 16, 6267 CrossRef PubMed
; (g) B. V. S. Reddy, M. Durgaprasad and B. Sridhar, J. Org. Chem., 2015, 80, 653 CrossRef PubMed
; (h) A. Venkateswarlu, M. Kanakaraju, A. C. Kunwar, Y. V. R. Reddy and B. V. S. Reddy, Eur. J. Org. Chem., 2015, 5389 CrossRef CAS
.
-
(a) H. M. Lee, C. N. Oberhuber and M. D. Shair, J. Am. Chem. Soc., 2008, 130, 16864 CrossRef CAS PubMed
; (b) E. Fenster, C. Fehl and J. Aube, Org. Lett., 2011, 13, 2614 CrossRef CAS PubMed
; (c) J. Lu, Z. Song, Y. Zhang, Z. Gan and H. Li, Angew. Chem., Int. Ed., 2012, 51, 5367 CrossRef CAS PubMed
; (d) M. R. Gesinski and S. D. Rychnovsky, J. Am. Chem. Soc., 2011, 133, 9727 CrossRef CAS PubMed
; (e) A. J. Bunt, C. D. Bailey, B. D. Cons, S. J. Edwards, J. D. Elsworth, T. Pheko and C. L. Willis, Angew. Chem., Int. Ed., 2012, 51, 3901 CrossRef CAS PubMed
; (f) B. D. Cons, A. J. Bunt, C. D. Bailey and C. L. Willis, Org. Lett., 2013, 15, 2046 CrossRef CAS PubMed
; (g) E. A. Crane and K. A. Scheidt, Angew. Chem., Int. Ed., 2010, 49, 8316 CrossRef CAS PubMed
; (h) X. Han, G. R. Peh and P. E. Floreancig, Eur. J. Org. Chem., 2013, 1193 CrossRef CAS
.
-
(a) Y. Yamamoto, Chem. Soc. Rev., 2014, 43, 1575 RSC
; (b) T. Kitamura, Eur. J. Org. Chem., 2009, 1111 CrossRef CAS
; (c) J. Carreras, G. Gopakumar, L. Gu, A. Gimeno, P. Linowski, J. Petuškova, W. Thiel and M. Alcarazo, J. Am. Chem. Soc., 2013, 135, 18815 CrossRef CAS PubMed
.
-
(a) M. Rudolph and A. S. K. Hashmi, Chem. Soc. Rev., 2012, 41, 2448 RSC
; (b) D. J. Gorin and D. Toste, Nature, 2007, 446, 395 CrossRef CAS PubMed
; (c) A. Stephen and K. Hashmi, Chem. Rev., 2007, 7, 3180 Search PubMed
; (d) Z. Li and C. Brower, Chem. Rev., 2008, 8, 3239 CrossRef PubMed
; (e) A. Arcadi, Chem. Rev., 2008, 8, 3266 CrossRef PubMed
; (f) E. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 8, 3326 CrossRef PubMed
; (g) D. Gorin, B. Sherry and F. D. Toste, Chem. Rev., 2008, 8, 3351 CrossRef PubMed
; (h) N. Marion and S. Nolan, Chem. Soc. Rev., 2008, 37, 1776 RSC
; (i) A. Stephen, A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766 RSC
.
-
(a) Y. Zhang, J. P. Donahue and C. J. Li, Org. Lett., 2007, 9, 627 CrossRef CAS PubMed
; (b) A. Arcadi, G. Bianchi, M. Chiarini and G. Marinelli, Synlett, 2004, 944 CrossRef CAS
; (c) A. Arcadi, M. Alfonsi, G. Bianchi, G. D'Anniballe and F. Marinelli, Adv. Synth. Catal., 2006, 348, 331 CrossRef CAS
; (d) M. Alfonsi, A. Arcadi, G. Bianchi and F. Marinelli, J. Org. Chem., 2005, 70, 2265 CrossRef CAS PubMed
.
-
(a) J. M. Lee, Y. Na, H. Han and S. Chang, Chem. Soc. Rev., 2004, 33, 302 RSC
; (b) J. Zhou, Chem.–Asian J., 2010, 5, 422 CrossRef CAS PubMed
.
- CCDC 1493462 contains supplementary crystallographic data for the structure 6j.†.
-
(a) K. Miura, K. Yamamoto, A. Yamanobe, K. Ito, H. Kinoshita, J. Ichikawa and A. Hosomi, Chem. Lett., 2010, 39, 766 CrossRef CAS
; (b) H. Kinoshita, C. Miyama and K. Miura, Tetrahedron Lett., 2016, 57, 5065 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra of products. CCDC 1493462. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra21375h |
| This journal is © The Royal Society of Chemistry 2016 |