DOI:
10.1039/C6RA21351K
(Paper)
RSC Adv., 2016,
6, 105338-105346
Ammonia synthesis and by-product formation from H2O, H2 and N2 by dielectric barrier discharge combined with an Ru/Al2O3 catalyst
Received
25th August 2016
, Accepted 13th October 2016
First published on 13th October 2016
Abstract
NH3 synthesis from H2O + N2, H2 + N2 or H2O + H2 + N2 by dielectric barrier discharge combined with an Ru/Al2O3 catalyst is studied at atmospheric pressure and room temperature. Additionally, the effects of reaction gas composition, energy density, and discharge frequency on NH3 yield and by-product formation are investigated. The results show that NH3 can be formed from reaction gases consisting of H2O and N2. The NH3 yield of the reaction gas containing H2 is much higher than that of the reaction gas consisting of H2O and N2. The presence of H2O has a promotion effect on NH3 synthesis from H2 and N2, especially for reaction gases with an H2 content less than 10%. The NH3 yield first increases and then decreases with an increase in the energy density. The maximum yield of 680 mg kW−1 h−1 occurs at the energy density of 1400 J L−1 and in the reaction gas of 0.14% H2O, 40% H2 and N2. The discharge frequency has a great effect on the NH3 yield, and maximum NH3 yield is obtained at the frequency of 13 kHz. Besides NH3, some by-products, such as N2O and NO2, are also formed with reaction gases containing H2O. However, their formation can be suppressed in the presence of H2, which is attributed to the reduction effect of H2 on the by-products.
1. Introduction
As the most important post treatment techniques for NOx emission from combustion processes, selective catalytic reduction (NH3-SCR) and selective non-catalytic reduction (NH3-SNCR) with NH3 as the reductant have gained wide attention both in the research field and in practical applications.1–4 Although their excellent denitration (DeNOx) performance has been generally recognized, the supply of NH3 remains a problem because of its toxicity and explosivity. To ensure safety, harsh measures have to be adopted for the transport, storage and usage of liquid and aqueous NH3. One effective approach to overcome these shortcomings is to generate NH3 onsite, where and when the need arises. Essentially, the on-board synthesis of NH3 for NOx removal in truck and car exhausts has already been investigated.5
The conventional ammonia synthesis technology, particularly the Haber–Bosch process, is only suitable for large scale and continuous production since it requires high operating temperatures (400–500 °C) and pressures (150–300 bar) over magnetite (Fe3O4) catalysts.6,7 Currently, the non-thermal plasma process, which is characterized by operating/stopping instantaneously, has emerged as an alternative method of treating air pollutants,8,9 as well as a potential way to synthesize gas products at atmospheric pressure and close to ambient gas temperature.10,11 Essentially, NH3 synthesis from N2–H2 gas mixtures induced by non-thermal plasma at ambient temperature was reported by Brewer as early as 1929.12 Currently, various forms of plasma, including microwave discharge plasma,13 radio frequency discharge plasma,14 ECR (electron cyclotron resonance) plasma,15 and expanding thermal plasma,7 are employed for the synthesis of NH3 from gas mixtures of N2 and H2. The apparatus for generating these types of plasma, however, operate under reduced pressure (several hundred Pa atmospheric pressure); thus they are unsuitable for practical applications.
Recently, the application of atmospheric pressure DBD plasma in NH3 synthesis was reported by Mizushima using a catalyst-loaded membrane as a dielectric material,16 Hong used a DBD packed bed reactor filled with dielectric spheres17 and Bai applied a microgap DBD reactor.18 Furthermore, Bai also pointed out that the NH3 yield increased by approximately 1.54–1.75 times when MgO powder was coated on the surface of the electrode plate.19 Improvement in NH3 yield was also achieved by loading the alumina tube with Ru, Pt, Ni, and Fe catalysts placed between the electrodes.20 Previous researchers generally thought that the reaction occurs on the surface of catalysts.21,22 Excited nitrogen molecules are absorbed on the surface of the catalyst and then dissociated to adsorbed nitrogen atoms, N (a). The nitrogen atoms, N (a), combine with hydrogen from the gas phase or on the surface of the catalyst to successively form NH (a) and NH2 (a) and finally become ammonia.22
In this study, we focus on the synthesis of NH3 from H2O, H2 and N2 by DBD-type plasma combined with an Ru/Al2O3 catalyst at atmospheric pressure and room temperature. Ru was selected as the catalyst and dielectric barrier material because it is seen as a second generation non-iron catalyst for NH3 synthesis from the reaction gases of H2 and N2.6,23 Moreover, H2O also acts as the source of hydrogen because hydrogen atoms can be produced by dissociating water molecules with the help of non-thermal plasma, in which abundant reactive species such as high-energy electrons, ions, excited atoms and molecules, and active radicals are contained. For this reason, a gas stream containing H2O, H2 and N2 as the reaction gases was used. Furthermore, the effects of reaction gas composition, energy density, and discharge frequency on NH3 yield are investigated.
2. Experimental
2.1 Experimental setup
A schematic of the experimental setup is shown in Fig. 1. It consists of a gas feeding system, a cylinder DBD reactor with an AC high-voltage power supply (Dalian University of Technology, China), a digital oscilloscope (Tektronix DPO3034, America) with a discharge parameter detection unit and a set of analytical instruments. H2 + N2, H2O + N2 or H2O + H2 + N2 were used as the reaction gases and the gas flow rates were controlled with mass flow controllers (MFC). H2O was introduced by bubbling N2 in deionized water at 25 °C and the relative humidity of the reaction gases was detected by a humidity meter (Cole Parmer, America). Then, the H2O content was calculated based on the relative humidity. The total gas flow rate was 500 mL min−1 except otherwise stated.
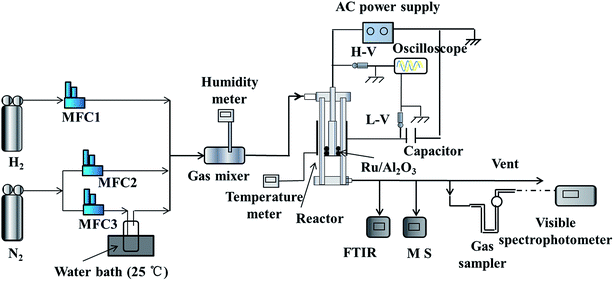 |
| | Fig. 1 Schematic of the experimental setup. | |
2.2 Plasma reactor
Fig. 2 shows a schematic of the concentric DBD reactor used in this study. An aluminium rod, which had the discharge length and outer diameter of 100 mm and 21 mm, respectively, served as the high-voltage electrode and was placed in a quartz tube with the length and inner diameter of 200 mm and 25 mm, respectively. Aluminum tape, wrapped on the outer surface of the quartz tube, was connected to a 10 nF capacitor before grounding. The Ru/Al2O3 catalyst was packed in the space between the high-voltage electrode and the quartz tube. By applying a high AC voltage, intense discharge plasma could be generated around the contact point of the catalyst without arcing.
 |
| | Fig. 2 Schematic of the DBD plasma reactor. | |
2.3 Preparation of Ru/Al2O3 catalyst
Aluminum oxide powder was provided by Aladdin Co. Ltd (Shanghai, China) and RuCl3 was obtained from Shenyang Jinke Reagent Co. Ltd (China). All chemicals were used as received without any further purification. Ru (1 wt%) was added by incipient wetness impregnation with an aqueous RuCl3 solution, followed by evaporation in a Rotavapor and drying (105 °C) in an oven overnight. Then, the as-prepared sample was reduced in hydrogen at 400 °C for 4 h to obtain Ru/Al2O3.
2.4 Characterization of the catalyst
X-ray powder diffraction (XRD) measurements were performed using a PANalytical X' Pert PRO diffractometer (Cu Pd radiation) at 40 kV and 40 mA. HRTEM micrographs were obtained with a JEM-2100F microscope at 200 kV. The surface chemical states of the Ru/Al2O3 catalyst were investigated via XPS (PHI Quantro SXM ULVAC-PHI, Japan) using an Al Kα X-ray source (1486.7 eV) at 15 kV and 25 W with the binding energy calibrated by C 1s at 284.8 eV.
2.5 Experimental method
NH3 was absorbed by dilute sulphuric acid and then quantitatively analyzed using a visible spectrophotometer based on Nessler's reagent colorimetric method.24 The other gas phase products were qualitatively confirmed using Fourier transform infrared spectrometry (FTIR, Nicolet 6700, America) and/or mass spectrometry (MS, OmniStar GSD300, Germany). The applied voltage and voltage drop through the capacitor were monitored with a high voltage probe (EP-50K, Japan) and a low voltage probe (Tektronix P5100, America), respectively. The Q–U Lissajous method was used to calculate the discharge power of the DBD reactor (P), which is defined as follows:where f, C and A are the frequency of discharge, capacitance of the external capacitor (10 nF) and the area of the Lissajous diagram, respectively.
Energy density (ED) is defined as the energy deposited per unit volume of reaction gas:
| |
 | (2) |
where
Q denotes the total gas flow rate in L min
−1.
In this study, the yield of NH3 (η, mg kW−1 h−1) is defined as follows:
| |
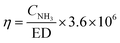 | (3) |
where
CNH3 (mg L
−1) is the concentration of NH
3 detected at the outlet of the reactor.
3. Results and discussion
3.1 Catalyst characterization
Fig. 3 shows the XRD spectra of the Ru/Al2O3 catalyst before and after the discharge reaction (5% H2 + N2, ED = 1400 J L−1). Both patterns contain only diffraction peaks from alumina. No peak correlated to Ru is observed, which is probably due to its relatively low content.
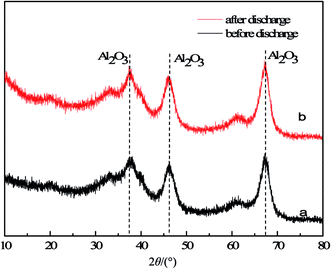 |
| | Fig. 3 X-ray diffraction patterns of Ru/Al2O3 catalyst: (a) before discharge and (b) after discharge (5% H2 + N2, ED = 1400 J L−1). | |
The distribution of Ru particles on the Al2O3 support was determined by TEM (Fig. 4), which shows that the Ru nanoparticles (∼3 nm) appear to be uniformly distributed on the Al2O3 support and the size and distribution of the Ru particles are almost unchanged after the discharge reaction.
 |
| | Fig. 4 Transmission electron micrograph of Ru/Al2O catalyst: (a) before discharge and (b) after discharge (5% H2 + N2, ED = 1400 J L−1). | |
XPS analysis was performed on the Ru/Al2O3 catalyst both before and after the discharge reaction (Fig. 5). The peak at 461.9 eV can be assigned as metallic Ru.25 As shown in Fig. 5, the valence state of Ru remains the same after the discharge reaction.
 |
| | Fig. 5 Ru 3p3/2 electron spectra of Ru/Al2O3 catalyst: (a) before discharge and (b) after discharge (5% H2 + N2, ED = 1400 J L−1). | |
3.2 Discharge characteristics
Fig. 6 shows the relationship between energy density and discharge voltage for different reaction gases at the same discharge frequency of 11.0 kHz. For the representative reaction gases, the energy density remains zero until the voltage reaches the discharge inception value and then the energy density dramatically increases with an increase in discharge voltage. The introduction of H2O results in an increase in the discharge inception value and a decrease in energy density at a given discharge voltage, which can be attributed to the strong electron affinities of the H2O molecules. The ability of H2O molecules to attach dissociative electrons is larger than that of H2.26 Therefore, a higher discharge inception voltage is needed to achieve the same energy density in the reactor for 0.14% H2O + N2, compared with the reaction gases consisting of 5% H2 + N2 or 0.14% H2O + 5% H2 + N2.
 |
| | Fig. 6 Energy density as a function of discharge voltage (f = 11.0 kHz). | |
3.3 Effect of H2O content on NH3 yield
3.3.1 Reaction gases consisting of H2O and N2. Fig. 7 illustrates the variation of NH3 yield with energy density for the H2O content of 0.07%, 0.14%, and 0.21%. For all the H2O contents, the NH3 yield first increases and then decreases with an increase in energy density, with maximum ammonia yields of 8.5 mg kW−1 h−1, 17.6 mg kW−1 h−1 and 16.1 mg kW−1 h−1 at energy densities of 500 J L−1, 670 J L−1 and 550 J L−1 for H2O content of 0.07%, 0.14%, and 0.21%, respectively. The NH3 yield enhances by about 2 times when the H2O content increases from 0.07% to 0.14%, whereas it almost remains the same for the H2O content of 0.14% and 0.21%.
 |
| | Fig. 7 Effect of H2O content on NH3 yield (reaction gases: H2O + N2, f = 11.0 kHz). | |
During discharge, dissociation processes can simultaneously occur due to inelastic collisions of H2O and N2 molecules with energetic electrons, which produce active species such as  , H2O*, N˙, H˙, ˙OH, O˙, N+, N2+ and H+ via the reactions (R1)–(R5), which can further react to form several types of products including NH3 (Fig. 7), H2 (Fig. 8) and NOx (Fig. 9).
, H2O*, N˙, H˙, ˙OH, O˙, N+, N2+ and H+ via the reactions (R1)–(R5), which can further react to form several types of products including NH3 (Fig. 7), H2 (Fig. 8) and NOx (Fig. 9).
 |
| | Fig. 8 Hydrogen ion current for different energy densities (reaction gases: 0.14% H2O + N2, f = 11.0 kHz). | |
 |
| | Fig. 9 FTIR spectra of the effluents after the DBD reaction (reaction gases: 0.14% H2O + N2, f = 11.0 kHz). | |
As can be seen from Fig. 8, the H2 yield, which is represented by ion current detected by MS, increases with an increase in energy density, thus indicating that more H atoms can be generated at a higher energy density, which explains the phenomenon that NH3 yield increases with an increase in energy density (Fig. 7). On the other hand, the FTIR results shown in Fig. 9 prove that NOx (mainly NO2 and N2O) is also produced during the DBD process and the concentration of NOx is greatly enhanced when the energy density is higher than a certain value. A portion of N species and discharge energy used for NH3 synthesis can be consumed in NOx formation (reactions (R6)–(R8)), which thus results in a decrease in NH3 yield with an increase in energy density after the peak point (Fig. 7). Furthermore, Mizushima pointed out that the occurrence of a backward reaction is a possible reason for the decrease in NH3 yield with an increase in energy density.16 In other words, at a higher energy density, more NH3 molecules are formed, and their decomposition to H2 and N2 is also promoted. For this reason, there exists an optimum energy density for obtaining a maximum NH3 yield in the plasma process.
| | |
N2 + e → N2+(X2∑g+, A2Πu, B2∑u+, C3∑u+) + 2e
| (R1) |
| | |
N2 + e → N+(3P) + N+(4S, 2D) + 2e
| (R2) |
| | |
N2+ + H2O → N2 + H2O+
| (R3) |
| | |
H2O+ + H2O → H3O+ + OH
| (R4) |
| | |
N + ˙OH → NO + H˙, k1 = 5.05 × 10−11 cm3 per molecule per s
| (R6) |
| | |
NO + O˙ → NO2, k2 = 3.01 × 10−11 cm3 per molecule per s
| (R7) |
| | |
NO2 + N → N2O + O, k3 = 1.2 × 10−11 cm3 per molecule per s
| (R8) |
3.3.2 Reaction gases consisting of H2O, H2 and N2. The effect of H2O content on NH3 yield is plotted in Fig. 10 when the basic composition of the reaction gases is 5% H2 and N2. With an increase in energy density from 0 to 2000 J L−1, the NH3 yield increases to a peak value and then decreases to a certain value regardless of the H2O content in the reaction gases. For the reaction gases consisting of 5% H2 and N2 (dry case), the maximum NH3 yield is 270 mg kW−1 h−1 at 1386 J L−1, which is much higher than the case for H2O and N2 (Fig. 7). The introduction of H2O to the reaction gases of 5% H2 and N2 results in an increase in the NH3 yield. For the same energy density of 1400 J L−1, the NH3 yield increases from 270 mg kW−1 h−1 (dry case) to 300 mg kW−1 h−1, 380 mg kW−1 h−1 and 310 mg kW−1 h−1 in the presence of 0.07% H2O, 0.14% H2O, and 0.21% H2O, respectively, which can be attributed to the formation of more H-containing species in favor of NH3 synthesis in the presence of H2O. Kikuchi studied the effect of H2O on atomic hydrogen generation in hydrogen plasma and proposed that the relative concentration of hydrogen atoms increases when water vapor is added.27
 |
| | Fig. 10 Effect of the addition of H2O on NH3 yield (basic reaction gases 5% H2 + N2, f = 11.0 kHz). | |
Fig. 11 shows the FTIR spectra of the effluents after the DBD reaction for different H2O contents under the same energy density of 1400 J L−1. It can be seen that the presence of H2O promotes NH3 formation. Moreover, only nitrous oxide (N2O) is detected as the byproduct and its concentration increases with an increase in H2O content. NO2, which is observed when H2O and N2 were used as the reaction gases (Fig. 9), is not detected in the presence of H2 (Fig. 11). This may be due to the fact that more NH and NH2 radicals exist in the discharge region as precursors of NH3. These free radicals are strongly reductive and can reduce NO and NO2 to N2O rapidly, as shown in eqn (R9)–(R11).
| | |
NO + NH → N2O + H˙, k4 = 8.39 × 10−1 cm3 per molecule per s
| (R9) |
| | |
NO2 + NH → N2O + ˙OH, k5 = 4.1 × 10−1 cm3 per molecule per s
| (R10) |
| | |
NO2 + NH2 → N2O + H2O, k6 = 1.4 × 10−1 cm3 per molecule per s
| (R11) |
 |
| | Fig. 11 FTIR spectra of the effluents after the DBD reaction (reaction gases: 5% H2 + H2O + N2, f = 11.0 kHz). | |
Evidently, the rate constants of these reduction reactions are several orders of magnitude higher than that of nitrogen oxide (NO and NO2) formation reactions (eqn (R6) and (R7)). As a result, the NO and NO2 formed during the plasma process are immediately consumed by the NH and NH2 radicals. On the other hand, an increase in the H2O content results in an increase in N2O concentration by providing more oxidizing species (e.g. O and OH) for NOx formation (eqn (R6) and (R7)), which may not be favorable for NH3 synthesis, and the NH3 yield decreases when the H2O content is higher than the optimum value of 0.14%.
3.4 Effect of H2 content on NH3 yield
3.4.1 Reaction gases consisting of H2 and N2. The effect of H2 content on NH3 yield without H2O is shown in Fig. 12. The NH3 yield increases to a peak value at an energy density of about 1400 J L−1 regardless of the H2 content. At this point, the NH3 yield reaches 270 mg kW−1 h−1, 440 mg kW−1 h−1, 620 mg kW−1 h−1, 640 mg kW−1 h−1, and 580 mg kW−1 h−1 for H2 contents of 5%, 10%, 20%, 40%, and 60%, respectively. In a traditional NH3 synthesis process, the H2 content of the reaction gases is usually around 75% (N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1
H2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). In this study, the observed optimum H2 content (40%) is lower than the calculated value from the stoichiometric ratio of H2 and N2 in NH3. Previous studies on NH3 synthesis in the presence of non-thermal plasma also indicate that the NH3 yield depends on the N2/H2 ratio in the reaction gases and a similar optimal ratio has been reported.19,28
3). In this study, the observed optimum H2 content (40%) is lower than the calculated value from the stoichiometric ratio of H2 and N2 in NH3. Previous studies on NH3 synthesis in the presence of non-thermal plasma also indicate that the NH3 yield depends on the N2/H2 ratio in the reaction gases and a similar optimal ratio has been reported.19,28
 |
| | Fig. 12 Effect of H2 content on NH3 yield (reaction gases: H2 + N2, f = 13 kHz). | |
Unlike the traditional NH3 synthesis process, in which molecules are dissociated on a catalytic surface at high temperatures (400–550 °C) and high pressures (15–35 MPa), under the non-thermal plasma condition, the precursor molecules, H2O, H2 and/or N2, are dissociated to N and H atoms. Thus, the surfaces of the catalyst are exposed to a high concentration of atomic N and H radicals. NH3 formation turns out to be strongly dependent on the content of atomic N and H radicals.15 When the H2 content is lower than the optimum H2/N2 ratio, the reaction possibility between H2 and N2 on the surface of the catalyst increases with an increase in H2 content, which is due to more reaction gases dissociated in favor of NH3 synthesis. However, if the H2 content further increases above the optimum value, NH3 synthesis is restrained mainly due to the following two reasons: firstly, more discharge power would be consumed for activating H2 molecules other than N2 molecules, since the dissociation energy of H2 (4.5 eV) is much lower than that of N2 (9.8 eV), which results in a shortage of nitrogen atoms for NH3 synthesis; secondly, with an increase in H2 content, more hydrogen atoms occupy the catalyst surface, thus reducing the number of adsorbed nitrogen atoms and also increasing the possibility of hydrogen atom recombination to hydrogen molecules.
3.5 Effect of discharge frequency on NH3 yield
As shown in Fig. 15, the discharge frequency has a great effect on the NH3 yield at a given energy density, with the maximum NH3 yield of 680 mg kW−1 h−1 at 13 kHz for reaction gases consisting of 0.14% H2O + 40% H2 + N2. For a discharge frequency lower than 13 kHz, an increase in discharge frequency results in an increase in microdischarge, which favors the formation of N- and H-containing species and the synthesis of NH3. However, further increase in the discharge frequency over 13 kHz results in a decrease in NH3 yield, possibly due to the dissociation of NH3 by the excessive energetic electrons. Thus, the NH3 yield decreases to 600 mg kW−1 h−1 at 14.5 kHz. These findings are in a fairly good agreement with Bai's experimental results.18
 |
| | Fig. 15 Effect of discharge frequency on NH3 yield (reaction gases: 0.14% H2O + 40% H2 + N2, ED = 1400 J L−1). | |
4. Reaction routes
Based on the results described above, we propose the reaction routes for the NH3 synthesis and by-product formation via a hybrid plasma-catalytic process, as shown in Fig. 16. It is well known that the rate-limiting step of NH3 synthesis in the conventional Haber–Bosch process is the dissociative chemisorption of N2 molecules on the surface of the catalyst and the dissociation process only occurs at a temperature between 400–500 °C due to the high binding energy of 9.6 eV of N2. In a DBD reactor, atomic N (a) species can be produced on the surface of the catalyst through the dissociative adsorption of  molecules excited by electron impacts at atmospheric pressure and room temperature. Plasma discharge also generates activated hydrogen species such as H atoms and
molecules excited by electron impacts at atmospheric pressure and room temperature. Plasma discharge also generates activated hydrogen species such as H atoms and  molecules. Then, NH3 can be produced by the reactions of N (a) atoms with H (a) atoms with the help of Ru/Al2O3. Essentially, this research shows that a small amount of NH3 (several mg per kW per h) is detected over alumina when the DBD reactor is on. The presence of Ru significantly promotes the production of NH3 (Fig. 16(a)).
molecules. Then, NH3 can be produced by the reactions of N (a) atoms with H (a) atoms with the help of Ru/Al2O3. Essentially, this research shows that a small amount of NH3 (several mg per kW per h) is detected over alumina when the DBD reactor is on. The presence of Ru significantly promotes the production of NH3 (Fig. 16(a)).
 |
| | Fig. 16 Reaction routes for NH3 synthesis and by-product formation by DBD-type plasma combined with the Ru/Al2O3 catalyst under different reaction gases: (a) H2 + N2; and (b) H2O + N2; (c) H2O + H2 + N2. | |
NH3, NO2, and N2O were formed from H2O and N2, because H2O is dissociated to give H and O atoms, and OH active species with the help of non-thermal plasma (Fig. 16(b)). For the reaction gas of H2O, H2 and N2, only N2O, as the by-product, was detected when the H2 content was less than 40% and N2O also disappeared when the H2 content was above 40% (Fig. 16(c)).
5. Conclusions
NH3 can be synthesized from reaction gases consisting of H2O + N2, H2 + N2 or H2O + H2 + N2 by DBD-type plasma combined with catalyst at atmospheric pressure and room temperature. The largest NH3 yields from H2 + N2 (640 mg kW−1 h−1) and H2O + H2 + N2 (680 mg kW−1 h−1) are much higher than that from H2O + N2 (18 mg kW−1 h−1), which is attributed to the fact that H2 molecules dissociate into hydrogen atoms more easily than H2O molecules.
The dissociation degrees of H2O, H2 and N2 enhance with an increase in energy density, which results in an increase in NH3 yield. However, energy densities higher than 500 J L−1 and 1400 J L−1 for the reaction gases of H2O + H2 and H2 + (H2O) + N2, respectively, are unfavorable for NH3 synthesis because the discharge power is more inclined to dissociate H2O and H2 instead of N2, which results in an N/H ratio below the optimal value.
Besides NH3, some by-products such as N2O and NO2 are also formed in the reaction gases containing H2O. However, their formation can be suppressed in the presence of H2, which is attributed to the reduction effect of H2 on the by-products. Furthermore, the by-products disappear for reaction gases containing higher than 40% H2.
Acknowledgements
The authors wish to thank Prof. A. Mizuno from the Toyohashi University of Technology for valuable discussions. The authors also gratefully acknowledge the financial support by the National Natural Science Foundation of China (No. 21377009 and 21547004).
References
- G. Busca, L. Lietti, G. Ramis and F. Berti, Appl. Catal., B, 1998, 18, 1–36 CrossRef CAS.
- W. Fan, T. Zhu, Y. Sun and D. Lv, Chemosphere, 2014, 113, 182–187 CrossRef CAS PubMed.
- M. T. Javed, N. Irfan and B. M. Gibbs, J. Environ. Manage., 2007, 83, 251–289 CrossRef CAS PubMed.
- L. Wang, H. Chen, M. Yuan, S. Rivillon, E. H. Klingenberg, X. Li and R. T. Yang, Appl. Catal., B, 2014, 152, 162–171 CrossRef.
- Y. Iitsuka, H. Yamauchi, S. Sato, K. Takashima, A. Mizuno and G. Prieto, IEEE Trans. Ind. Appl., 2012, 48, 872–877 CrossRef CAS.
- N. Saadatjou, A. Jafari and S. Sahebdelfar, Chem. Eng. Commun., 2015, 202, 420–448 CrossRef CAS.
- R. Michalsky, B. J. Parman, V. Amanor-Boadu and P. H. Pfromm, Energy, 2012, 42, 251–260 CrossRef CAS.
- S. Samukawa, M. Hori, S. Rauf, K. Tachibana, P. Bruggeman, G. Kroesen, J. C. Whitehead, A. B. Murphy, A. F. Gutsol and S. Starikovskaia, J. Phys. D: Appl. Phys., 2012, 45, 1–5 CrossRef.
- X. Zhu, X. Gao, R. Qin, Y. Zeng, R. Qu, C. Zheng and X. Tu, Appl. Catal., B, 2015, 170–171, 293–300 CrossRef CAS.
- X. Tu and J. C. Whitehead, Appl. Catal., B, 2012, 125, 439–448 CrossRef CAS.
- K. Zhang, B. Eliasson and U. Kogelschatz, Ind. Eng. Chem. Res., 2002, 41, 1462–1468 CrossRef CAS.
- A. K. Brewer and J. W. Westhaver, J. Phys. Chem., 1930, 34, 153–164 CrossRef.
- H. Uyama and O. Matsumoto, Plasma Chem. Plasma Process., 1989, 9, 421–432 CrossRef CAS.
- H. Uyama and O. Matsumoto, Plasma Chem. Plasma Process., 1989, 9, 13–24 CrossRef CAS.
- H. Uyama and O. Matsumoto, Plasma Chem. Plasma Process., 1996, 16, 547–562 CrossRef.
- T. Mizushima, K. Matsumoto, J. I. Sugoh, H. Ohkita and N. Kakuta, Appl. Catal., A, 2004, 265, 53–59 CrossRef CAS.
- J. Hong, S. Prawer and A. B. Murphy, IEEE Trans. Plasma Sci., 2014, 42, 2338–2339 CrossRef.
- M. Bai, Z. Zhang, X. Bai, M. Bai and W. Ning, IEEE Trans. Plasma Sci., 2003, 31, 1285–1291 CrossRef CAS.
- M. Bai, X. Bai, Z. Zhang and M. Bai, Plasma Chem. Plasma Process., 2000, 20, 511–520 CrossRef.
- T. Mizushima, K. Matsumoto, H. Ohkita and N. Kakuta, Plasma Chem. Plasma Process., 2007, 27, 1–11 CrossRef CAS.
- J. H. van Helden, W. Wagemans, G. Yagci, R. A. B. Zijlmans, D. C. Schram, R. Engeln, G. Lombardi and G. D. Stancu, J. Appl. Phys., 2007, 101, 101–112 CrossRef.
- K. Sugiyama, K. Akazawa, M. Oshima, H. Miura, T. Matsuda and O. Nomura, Plasma Chem. Plasma Process., 1986, 6, 179–193 CrossRef CAS.
- W. Rarog, Z. Kowalczyk, J. Sentek, D. Skladanowski and J. Zielinski, Chem. Lett., 2000, 68, 163–168 CAS.
- GB/T 14668-93, Air quality-determination of ammonia-Nessler's reagent colorimetric method.
- F. Sen and G. Gokagac, Energy Fuels, 2008, 22, 1858–1864 CrossRef CAS.
- A. Fridman, in Plasma Chemistry, Cambridge University, New York, 2008, ch. 2, p. 33 Search PubMed.
- J. Kikuchi, S. Fujimura, M. Suzuki and H. Yano, Jpn. J. Appl. Phys., 1993, 32, 70–76 Search PubMed.
- J. Nakajima and H. Sekiguchi, Thin Solid Films, 2008, 516, 4446–4451 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 
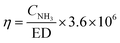



 , H2O*, N˙, H˙, ˙OH, O˙, N+, N2+ and H+ via the reactions (R1)–(R5), which can further react to form several types of products including NH3 (Fig. 7), H2 (Fig. 8) and NOx (Fig. 9).
, H2O*, N˙, H˙, ˙OH, O˙, N+, N2+ and H+ via the reactions (R1)–(R5), which can further react to form several types of products including NH3 (Fig. 7), H2 (Fig. 8) and NOx (Fig. 9).


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 1
H2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). In this study, the observed optimum H2 content (40%) is lower than the calculated value from the stoichiometric ratio of H2 and N2 in NH3. Previous studies on NH3 synthesis in the presence of non-thermal plasma also indicate that the NH3 yield depends on the N2/H2 ratio in the reaction gases and a similar optimal ratio has been reported.19,28
3). In this study, the observed optimum H2 content (40%) is lower than the calculated value from the stoichiometric ratio of H2 and N2 in NH3. Previous studies on NH3 synthesis in the presence of non-thermal plasma also indicate that the NH3 yield depends on the N2/H2 ratio in the reaction gases and a similar optimal ratio has been reported.19,28
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−16
exp(−16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/RT) cm3 per molecule per s
000/RT) cm3 per molecule per s

 molecules excited by electron impacts at atmospheric pressure and room temperature. Plasma discharge also generates activated hydrogen species such as H atoms and
molecules excited by electron impacts at atmospheric pressure and room temperature. Plasma discharge also generates activated hydrogen species such as H atoms and  molecules. Then, NH3 can be produced by the reactions of N (a) atoms with H (a) atoms with the help of Ru/Al2O3. Essentially, this research shows that a small amount of NH3 (several mg per kW per h) is detected over alumina when the DBD reactor is on. The presence of Ru significantly promotes the production of NH3 (Fig. 16(a)).
molecules. Then, NH3 can be produced by the reactions of N (a) atoms with H (a) atoms with the help of Ru/Al2O3. Essentially, this research shows that a small amount of NH3 (several mg per kW per h) is detected over alumina when the DBD reactor is on. The presence of Ru significantly promotes the production of NH3 (Fig. 16(a)).









