One-step fabrication of high quantum yield sulfur- and nitrogen-doped carbon dots for sensitive and selective detection of Cr(VI)†
Saihua Wangab,
Hongyun Niu*a,
Sijing Hea and
Yaqi Caia
aState Key Laboratory of Environmental Chemistry, Ecotoxicology of Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China. E-mail: hyniu@rcees.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 4th November 2016
Abstract
In this work, we have developed a green, simple, and low-cost hydrothermal method using wool and pig hair as the precursor to fabricate sulfur- and nitrogen-doped carbon dots (CDs). The precursors are natural and nontoxic raw materials, and the one-step strategy requires no organic solvents. The as-prepared carbon dots exhibit good water dispersibility, strong fluorescence emission with a relatively high quantum yield of 25.6% (contributed by the doped N and S elements), excellent pH stabilities and high ionic strength tolerance. More importantly, the fluorescence intensity of the CDs could be significantly and selectively quenched in the presence of Cr(VI) due to the oxidation–reduction reaction between Cr(VI) and the oxygen-containing groups and S-related species on the surface of CDs. Accordingly, the CDs are employed as a fluorescent probe for the detection of Cr(VI) ions in water. This CDs sensor exhibits high sensitivity to Cr(VI) with a limit of detection of 16.8 nM in a wide range of 50 nM to 100 μM. Furthermore, the sensor is successfully applied in the detection of Cr(VI) in real water samples.
Introduction
Chromium is one of the major water pollutants due to its widespread use in modern industries, including leather tanning, electroplating and wood preservation. Consequently, chromium has inevitably led to serious threats to the environment and human health. Cr(VI) is most hazardous because of its highly carcinogenic and mutagenic properties.1 So, an accurate and reliable method for the determination of Cr(VI) is in great demand. Until now, numerous analytical techniques, including chromatography,2 spectrophotometry,3 electrochemical techniques4 and atomic absorption spectrometry,5 have been developed for the direct assay of Cr(VI) ions. However, most of these techniques require expensive equipment and complicated pretreatment steps. Therefore, spectrophotometry with fluorescent probes has been a great alternative for its quick, simple and highly sensitive properties. But the fluorescent probes used to detect Cr(VI) in previous reports always are small organic dyes,3 organic nanoparticles,6 or semiconductor quantum dots,7 which have intrinsic limitation, such as low sensitivity, photobleaching effect, broad emission bands and biotoxicity.Carbon dots (CDs), a new member of carbon nanomaterials, have remarkable advantage in high resistance to photobleaching, robust chemical inertness, high sensitivity, low toxicity and good biocompatibility.8–11 Due to the outstanding properties, CDs have been used as fluorescent probes for the trace analysis of metal ions (such as Fe3+, Hg2+, Cu2+ and Ag+, etc.),12–17 and some organic compounds (such as glucose, DNA, vitamin B12, and dopamine, etc.).18–21 Recently, graphene quantum dot membrane,22 boron and nitrogen co-doped carbon dots,23 cobalt(II)-doped carbon dots,24 and phosphate functionalized carbon dots25 have all been successfully employed as probes for the determination of Cr(VI). But as far as we know, the fluorescent probes for Cr(VI) in previous reports have involved organic reagent, complex process and severe synthetic conditions. The desire for novel and simple methods to fabricate sulfur and nitrogen co-doped CDs which are highly sensitive for the detection of Cr(VI) ions is still challenging.
To overcome these shortcomings, several researchers have devoted to the exploring of new green and simple methods without the use of organic chemicals in recent years. Among them, green synthetic approaches using cheap and eco-friendly biomass directly from nature such as green tea,26 milk,27 garlic,28 soya bean,29 watermelon peel30 and honey31 as the precursors to produce CDs are very promising solutions and have drawn a great deal of attention. However, the reported CDs derived from natural plant precursor abundant in saccharide usually have either low quantum yield (QY) or low pH stabilities, which limit their application in environmental analysis. Therefore, it is highly desirable to explore a new carbon source for the economical and green synthesis of efficient fluorescent CDs.
Recently, Hou applied a hydrothermal method to prepare CDs with human hair, mainly consisting of carbon, oxygen and nitrogen owing to the large amount of keratin, as precursor. The obtained CDs exhibited strong and stable PL, which is dependent on excitation wavelength and pH, high quantum yield, and high sensitivity and good selectivity to the determination of Hg2+.12 Zhu developed a novel one-step approach for the large-scale synthesis of sulfur- and nitrogen-co-doped carbon dots (S–N–C-dots) with tunable luminescence properties by using sulfuric acid carbonization and etching of hair fiber.32 In the present study, the nature precursors for CDs were expanded to animal hair (wool and pig hair), which are much cheaper and less influenced by chemicals like shampoos, hair dyes and so on. The synthesis method has the advantage of green, simple and low cost. The achieved CDs are rich in sulfur and nitrogen elements, and possess good water dispersibility, strong fluorescence emission, brilliant pH stabilities and high ionic strength tolerance. Most importantly, the as-synthesized CDs exhibit excellent selectivity to Cr(VI).
Experimental section
Materials
The wool and pig hair were collected from a farm. K2Cr2O7, K2CrO4, NaCl, KCl, AgNO3, MgCl2·6H2O, MnCl2·4H2O, CaCl2, FeCl2·4H2O, FeCl3·6H2O, BaCl2·2H2O, CoCl2·6H2O, Cu(NO3)2·3H2O, Hg(NO3)2, Pb(NO3)2, CrCl3, Zn(Ac)2·2H2O, CdCl3·6H2O, NaClO4, NaBr, Na2SO4, KH2PO4, EDTA and KSCN were purchased from Sinopharm Chemical Reagent Co., Ltd (China). Quinine sulfate dihydrate ((C20H24N2O2)2·H2SO4·2H2O) was obtained from J&K Chemicals Ltd. (Beijing, China). All chemicals were of analytical reagent grade and used without further purification. Ultrapure water was prepared in the lab using a Milli-Q SP reagent water system (Millipore, Bedford, MA, USA).Instruments and characterizations
The fluorescence (FL) and the UV-vis absorption spectra were performed with a Hitachi F-4600 fluorescence spectrophotometer and Hitachi UV-2910 spectrophotometer, respectively. A JEM-2100 field-emission transmission electron microscopy (JEOL, Japan) was used to characterize the morphology of the as-prepared CDs. The crystal structure was obtained by X-ray diffraction (XRD, PAN-alytical X'Pert diffractometer, Almelo, Netherlands) using a monochromatized X-ray beam with nickel-filtered Cu Kα radiation and 4° min−1 scan rate. Fourier transform infrared (FTIR) spectra were recorded using KBr pressed pellets on a NEXUS 670 Infrared Fourier Transform spectrometer (Nicolet Thermo, USA). X-ray photoelectron spectroscopy (XPS) measurements were conducted by applying a Thermo Scientific ESCA-LAB-200i-XL spectrometer (Waltham, MA) with monochromatic Al Kα radiation (1486.6 eV).Synthesis of the carbon dots
The carbon dots were synthesized using a hydrothermal carbonization method. The wool and pig hair were firstly washed with cleanser essence and distilled water thoroughly, cut into small pieces (∼5 mm-in-length), and dried in oven. Then 0.5 g prepared fibers and 10 mL ultrapure water were transferred into a 25 mL Teflon-lined stainless steel autoclave, heated at 240 °C for 6 h, and cooled to room temperature naturally. Next, the aqueous solution was filtered through a 0.22 μm membrane and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min to discard the non-fluorescent deposits. The bright yellow CDs aqueous solution was collected and further dialyzed in a dialysis bag (molecular weight cutoff = 1000 Da) for 24 h. The purified CDs were preserved at 4 °C for further use. The CDs prepared from wool and pig hair are named as W-CDs and P-CDs, respectively.
000 rpm for 10 min to discard the non-fluorescent deposits. The bright yellow CDs aqueous solution was collected and further dialyzed in a dialysis bag (molecular weight cutoff = 1000 Da) for 24 h. The purified CDs were preserved at 4 °C for further use. The CDs prepared from wool and pig hair are named as W-CDs and P-CDs, respectively.
Fluorescence detection of Cr(VI) ions
For a typical assay, 3 μL of CDs, followed by the addition of different concentrations of Cr(VI) ions, were diluted to 1 mL with deionized water by a vortex mixer for a few seconds. The mixtures were equilibrated at room temperature for 20 min before the FL spectroscopy measurements were recorded. The resulting solutions were measured by FL spectroscopy at room temperature with an excitation at 350 nm, both the excitation and emission slit were 3 nm.Selectivity measurements
To examine the selectivity of CDs to the Cr(VI) anions, a series of competitive metal ions and anions, including Na+, Ca2+, Cd2+, Cr3+, K+, Fe3+, Fe2+, Ag+, Mg2+, Ba2+, Zn2+, Cu2+, Pb2+, Mn2+, Co2+, Hg2+, Cl−, Br−, SO42−, H2PO4−, Ac− and ClO4− were mixed with 3 μL of CDs by a vortex mixer under the same conditions. The concentration of Cr(VI) ions and other interference ions were 500 μM. The resulting solutions were measured by FL spectroscopy at room temperature with excitation at 350 nm, both the excitation and emission slit widths were 3 nm.Real samples
Three types of environmental water samples, namely lake water sample, tap water sample and river water sample, were used to evaluate the effect of sample matrix on Cr(VI) detection. The lake water sample was acquired from Gaobeidian Lake, Beijing, China. The river sample was collected in Xiaoyue River, Beijing, China. The tap water sample was taken from our laboratory in Haidian District, Beijing. All samples were collected randomly and filtered through 0.22 μm nylon membranes to remove suspended particles. The filtered water samples were analyzed within 24 h.Results and discussion
Optimization of synthesis conditions
As the fluorescent behavior of the carbon dots is affected by the mass of the precursor, the temperature and time of the hydrothermal process, it's necessary to optimize the experimental conditions in order to achieve carbon dots with the best fluorescence properties. As showed in Fig. S1A,† the FL QY of W-CDs increased with the increase of wool mass in the precursor solution (10 mL ultrapure water) ranging in 0.1 to 0.5 g and then decreased as the wool mass in the precursor solution went over 0.5 g. So the wool mass was fixed at 0.5 g, then the effect of temperature was investigated. The result clearly demonstrated that the FL QY of W-CDs increased gradually with the temperature and obtained the highest QY at 240 °C (Fig. S1B).† According to the Fig. S1C,† the FL QY of W-CDs increased with the increase of reaction time up to 6 h and decreased in the period from 6 to 12 h. In summary, the optimum experimental conditions are as follows: wool or pig hair mass 0.5 g, reaction temperature 240 °C and reaction time 6 h.Structure characterization
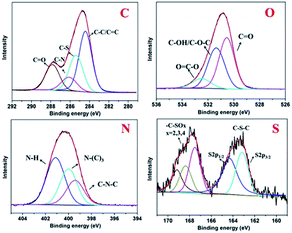
The morphology and structure of the as-prepared fluorescent CDs were characterized by HRTEM. The as-prepared CDs have spherical shapes and are well dispersed in water solution (Fig. 1 and S2†). Furthermore, well-defined lattice fringes are clearly observed (inset in Fig. 1A), with a lattice spacing of 0.20 nm, corresponding to the (102) diffraction plane of graphitic (sp2) carbon. As estimated from the HRTEM image, the W-CDs exhibited a narrow size distribution in the range of 4–8 nm with an average size of 5.9 nm (Fig. 1B). The XRD pattern of W-CDs displayed a broader (002) peak centered at around 24.5°, which further confirmed the graphene structure of the W-CDs (Fig. S3A†).The chemical structure of CDs was further characterized by X-ray photoelectron spectroscopy (XPS). In Fig. S3B,† the chemical compositions of W-CDs consisted of C, O, N and S, and the contents of these atoms were 62.54, 23.05, 13.66 and 0.75%, respectively. The high resolution C 1s, O 1s, N 1s and S 2p XPS spectra were shown in Fig. 2. The C 1s spectrum can be resolved as follows: C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 284.4 eV, C–S at 285.4 eV, C–N at 286.3 eV, and C
C at 284.4 eV, C–S at 285.4 eV, C–N at 286.3 eV, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 287.9 eV. The three fitted peaks at 530.5, 531.4 and 532.5 eV in O 1s spectrum were assigned to C
O at 287.9 eV. The three fitted peaks at 530.5, 531.4 and 532.5 eV in O 1s spectrum were assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH/C–O–C and O
O, C–OH/C–O–C and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups, respectively. The N 1s peaks at 399.4, 400.0 and 401.1 eV indicated that nitrogen existed mostly in the form of C–N–C, N–C3 and N–H, respectively. The S 2p spectrum consisted of two peaks, corresponding to C–S–C (163.6 eV) and –C–SOx– (x = 2, 167.5 eV; x = 3, 168.4 eV, x = 4, 169.1 eV), respectively.14 These indicated that the as-prepared W-CDs were rich in hydrophilic groups (hydroxyl, carbonyl, and carboxylic acid groups) on the surfaces. The presence of these functional groups endowed excellent solubility to the CDs in water without further chemical modification.
C–O groups, respectively. The N 1s peaks at 399.4, 400.0 and 401.1 eV indicated that nitrogen existed mostly in the form of C–N–C, N–C3 and N–H, respectively. The S 2p spectrum consisted of two peaks, corresponding to C–S–C (163.6 eV) and –C–SOx– (x = 2, 167.5 eV; x = 3, 168.4 eV, x = 4, 169.1 eV), respectively.14 These indicated that the as-prepared W-CDs were rich in hydrophilic groups (hydroxyl, carbonyl, and carboxylic acid groups) on the surfaces. The presence of these functional groups endowed excellent solubility to the CDs in water without further chemical modification.
Meanwhile, the FTIR spectrum of CDs demonstrates the presence of the same groups as indicated by the XPS results (Fig. 3). The peak at 1135 cm−1 was ascribed to the C–O and C–S bonds. The peak at 1408 cm−1 can be ascribed to the –COO group while the peak at 1629 cm−1 was ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and the peak at 2070 cm−1 was assigned to the C–N group. In addition, the broad band at 3424 cm−1 corresponded to the stretching modes of N–H and O–H. In short, these results confirmed that the obtained CDs are nitrogen-rich and sulfur-doped as expected.
O stretching vibration and the peak at 2070 cm−1 was assigned to the C–N group. In addition, the broad band at 3424 cm−1 corresponded to the stretching modes of N–H and O–H. In short, these results confirmed that the obtained CDs are nitrogen-rich and sulfur-doped as expected.
Optical characterization
The optical properties of the CDs were studied using UV-vis absorption and photoluminescence spectroscopy. As presented in Fig. S4A,† the resultant W-CDs displayed two typical UV-vis absorption peaks at ∼272 and 340 nm, respectively. The broad absorption peak around 272 nm was ascribed to the π–π* transition of aromatic sp2 domains, and the absorption peak centered at 340 nm indicated the presence of carbonyl or conjugated carbonyl groups.33 At the same time, the excitation and emission peaks appeared at 350 nm and 430 nm, respectively. As illustrated in Fig. S4B,† the emission from W-CDs gradually shifted to higher wavelengths when the excitation wavelength increased from 300 to 400 nm. The emission intensity increased when the excitation wavelength changed from 300 to 350 nm and then decreased gradually. This indicates the excitation-dependent emission behavior of W-CDs, which is one of the most special properties of carbon dots. The obtained W-CDs displayed narrow and symmetrical fluorescence spectra profiles, resulting from their narrower size distribution. Furthermore, the doping of N and S atoms significantly increased the quantum yield of the CDs, and the fluorescence quantum yield of W-CDs and P-CDs was calculated to be 25.6% and 20.1%, respectively. As a result, the CDs were able to perform competently as a highly sensitive chemosensor.The stability of CDs were investigated by measuring the FL intensity of the CDs under extreme pH and high ionic strength in solution. Fig. S5A† showed that the FL of the W-CDs at 430 nm upon excitation at 350 nm was strong and stable over a wide range of pH values (pH 2–10). As solution pH reached to 12, the FL intensity was only decreased by 35%. In the presence of different concentrations of NaCl (up to 1 M), the FL intensities remained constant with the increase of ionic strength, indicating the high stability of the W-CDs under a high ionic strength environment (Fig. S5B†). Furthermore, the FL intensity had no obvious decrease as the sample was stored for over 60 days (Fig. S5C†). Similar to the W-CDs, P-CDs also showed high pH stabilities and ionic strength tolerance. In conclusion, these finding suggested that the CDs were highly stable under extreme conditions and had great potential for sensing applications.
Fluorescence response of CDs toward Cr(VI)
The as-prepared CDs were used to detect Cr(VI) in water. The pH value of the solution is an important factor that affects the quenching efficiency. There is no significant difference in the quenching efficiency (F/F0) in the presence of Cr(VI) in the range of pH 2–10 (Fig. S6A†). This indicates that the determination of Cr(VI) with CDs as fluorescent sensor is not pH-dependent. In addition, the kinetic characteristics of the reaction system was investigated. The reaction was complete within 1 min, and the stable fluorescent signal at 430 nm remained constant for at least 1 h (Fig. S6B†). Under the optimized conditions, the strong emission of the CDs could be quenched obviously when Cr(VI) ions were added into the solution. As shown in Fig. 4, upon the addition of Cr(VI), the fluorescence intensity at 430 nm decreased with the increase of the Cr(VI) concentration. There was a good linear relationship (R2 = 0.997) between the quenching efficiency (F/F0) and the concentration of Cr(VI) in the range of 0.05–100 μM (Fig. 4 inset), where F0 and F are the FL intensity at 430 nm in the absence and presence of Cr(VI), respectively. The limit of detection (LOD) for Cr(VI) ions was estimated to be 16.8 nM at a signal-to-noise ratio of 3. Similar with the W-CDs, the P-CDs could selectively detect the Cr(VI) ions in the range of 0.05–100 μM as well, and the limit of detection for Cr(VI) was 29.6 nM (Fig. S7†). The above results suggested that the CDs were robust and fast fluorescent probes for the detection of Cr(VI) in water.To estimate the selectivity of CDs as a fluorescent probe, the effects of different kinds of competitive metal ions on the fluorescence were investigated, including Na+, Ca2+, Cd2+, Cr3+, K+, Fe3+, Fe2+, Ag+, Mg2+, Ba2+, Zn2+, Cu2+, Pb2+, Mn2+, Co2+, Hg2+ at a concentration of 500 μM. With the exception of Fe3+, Ag+ and Hg2+ cations, most of the metal ions including Cr3+ would not interfere the detection of Cr(VI) (Fig. 5A). We used KSCN as Ag+ ions chelator and EDTA as Fe3+ and Hg2+ ion chelator to eliminate the interference of these cations to the detection of Cr(VI). As a result, the interference of the Ag+, Fe3+ and Hg2+ ions were negligible in the presence of KSCN and EDTA (Fig. 5B). Cr(VI) is anion (CrO42− or Cr2O72−) in water solution, so we also investigated the effect of some common anions. The results showed that Ac−, SO42−, Cl− and Br− can not quench the fluorescence of the W-CDs, ClO4− and H2PO4− even slightly enhance the fluorescence of the W-CDs at the concentration of 500 μM (Fig. 5C). The P-CDs show the similar trend toward Cr(VI) as well (Fig. S8†). All these results clearly demonstrated that the present fluorescent sensor CDs exhibits high selectivity for the Cr(VI) detection in water environmental samples.
Real samples testing
The as-prepared CDs sensor was applied to the detection of Cr(VI) in real water samples by the standard addition analysis. The results were listed in Table 1. The recovery of the added known amount of Cr(VI) ions to the three water samples was in the range of 94.6–108% and 96.0–104%, respectively; and the relative standard deviation (RSD) was in the range of 1.4–5.3% and 0.91–4.3%, respectively, indicating the present CDs can be an excellent sensor for the direct determination of Cr(VI) for real samples analysis (Fig. 6).| Sample | Added Cr(VI) ions (μM) | Measured (μM) | Recovery (%) | RSD (n = 3, %) | |||
|---|---|---|---|---|---|---|---|
| W-CDs | P-CDs | W-CDs | P-CDs | W-CDs | P-CDs | ||
| Tap water | 0.5 | 0.48 | 0.51 | 95.5 | 101 | 5.3 | 1.8 |
| 50 | 48.26 | 49.42 | 96.5 | 98.8 | 1.4 | 2.1 | |
| Lake water | 0.5 | 0.47 | 0.50 | 94.6 | 99.9 | 3.0 | 4.3 |
| 50 | 52.05 | 52.16 | 104 | 104 | 4.9 | 0.91 | |
| River water | 0.5 | 0.51 | 0.48 | 103 | 96.0 | 2.4 | 3.3 |
| 50 | 54.12 | 51.15 | 108 | 102 | 4.8 | 1.8 | |
Possible mechanism of the FL quenching of CDs to Cr(VI)
The fluorescence quenching of W-CDs and P-CDs to Cr(VI) is believed to be related to the formation of a new complex and fast electron transfer between Cr(VI) and CDs. According to the XPS results, the peak intensity of oxygen containing functional groups is different from that in carbon dots before the addition of Cr(VI). The ratio of the –COOH peak increased, the ratio of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak decreased, and the S 2p band shifted to a high binding energy. These results are due to the fact that chromium might bind to groups containing oxygen, such as carboxyl or phenolic groups present on the surface of carbon dots,11 meanwhile, Cr(VI) adsorbed on the surface of CDs can coordinate with thiol groups on edges of CDs. The changes of the peaks are attributed to the reduction of the adsorbed Cr(VI) ions, with a simultaneous formation of additional carboxylic groups on the surface of carbon dots, which can act as binding sites for Cr(III).12 Furthermore, the spectra of Cr were also detected. In pure K2Cr2O7, the peaks at 579.7 and 588.9 eV were assigned to the Cr 2p1/2 and Cr 2p3/2 groups of Cr(VI), respectively. After reaction with W-CDs, the Cr element was detected on the surface of W-CDs. But, the peaks at 577.2 and 586.6 eV were assigned to the groups of Cr(III).34,35 The presence of Cr(III) is thought to originate from the chemical interactions of Cr(VI) ions with carbon dots. This clearly demonstrates that oxidation–reduction reaction has occurred between Cr(VI) and W-CDs, which generated electron transfer from the CDs to the Cr(VI) and led to nonradiative electron/hole recombination, resulting in the fluorescence quenching.
O peak decreased, and the S 2p band shifted to a high binding energy. These results are due to the fact that chromium might bind to groups containing oxygen, such as carboxyl or phenolic groups present on the surface of carbon dots,11 meanwhile, Cr(VI) adsorbed on the surface of CDs can coordinate with thiol groups on edges of CDs. The changes of the peaks are attributed to the reduction of the adsorbed Cr(VI) ions, with a simultaneous formation of additional carboxylic groups on the surface of carbon dots, which can act as binding sites for Cr(III).12 Furthermore, the spectra of Cr were also detected. In pure K2Cr2O7, the peaks at 579.7 and 588.9 eV were assigned to the Cr 2p1/2 and Cr 2p3/2 groups of Cr(VI), respectively. After reaction with W-CDs, the Cr element was detected on the surface of W-CDs. But, the peaks at 577.2 and 586.6 eV were assigned to the groups of Cr(III).34,35 The presence of Cr(III) is thought to originate from the chemical interactions of Cr(VI) ions with carbon dots. This clearly demonstrates that oxidation–reduction reaction has occurred between Cr(VI) and W-CDs, which generated electron transfer from the CDs to the Cr(VI) and led to nonradiative electron/hole recombination, resulting in the fluorescence quenching.
Conclusions
In summary, a green, simple, and low-cost hydrothermal method using wool and pig hair as the precursor has been developed to fabricate sulfur- and nitrogen-doped carbon dots for the first time. The precursors come from natural raw material, which are nontoxic and readily available. The obtained CDs have desirable functional groups attached to the particle surface and exhibit strong fluorescence emission, excellent pH stabilities and high ionic strength tolerance. The robust CDs exhibit high selectivity to Cr(VI) over most coexisting metal cations and anions. By employing this sensor, the quenching degree of the CDs is linearly proportional to the concentrations of Cr(VI) ions in a wide range of 50 nM to 100 μM and the detection limit is in the range of nM. In conclusion, the easily synthesized sulfur- and nitrogen-doped carbon dots can serve as a very efficient fluorescent sensor for the detection of Cr(VI).Acknowledgements
This work was jointly supported by National Basic Research Program of China (2015CB932003), the National Natural Science Foundation of China (21537004, 21277152, 21277002, 21477140), and Strategic Priority Research Program of the Chinese Academy of Sciences (XDB14010201).Notes and references
- J. Johnson, L. Schewel and T. E. Graedel, Environ. Sci. Technol., 2006, 40, 7060–7069 CrossRef CAS PubMed.
- V. Arancibia, M. Valderrama, K. Silva and T. Tapia, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2003, 785, 303–309 CrossRef CAS.
- Y. Xiang, L. Mei, N. Li and A. Tong, Anal. Chim. Acta, 2007, 581, 132–136 CrossRef CAS PubMed.
- R. Ouyang, S. A. Bragg, J. Q. Chambers and Z. L. Xue, Anal. Chim. Acta, 2012, 722, 1–7 CrossRef CAS PubMed.
- M. R. Moghadam, S. Dadfarnia and A. M. H. Shabani, J. Hazard. Mater., 2011, 186, 169–174 CrossRef CAS PubMed.
- M. Hosseini, V. K. Gupta, M. R. Ganjali, Z. Rafiei-Sarmazdeh, F. Faridbod, H. Goldooz, A. R. Badiei and P. Norouzi, Anal. Chim. Acta, 2012, 715, 80–85 CrossRef CAS PubMed.
- C.-X. Sui, Y.-F. Liu, W.-H. Zhang, P.-A. Li and D. Zhang, Microchim. Acta, 2013, 181, 347–353 CrossRef.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921 RSC.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230 RSC.
- H. Ding, J. S. Wei and H. M. Xiong, Nanoscale, 2014, 6, 13817–13823 RSC.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed. Engl., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- J. Hou, J. Li, J. Sun, S. Ai and M. Wang, RSC Adv., 2014, 4, 37342 RSC.
- Z. X. Wang and S. N. Ding, Anal. Chem., 2014, 86, 7436–7445 CrossRef CAS PubMed.
- Y.-C. Lu, J. Chen, A.-J. Wang, N. Bao, J.-J. Feng, W. Wang and L. Shao, J. Mater. Chem. C, 2015, 3, 73–78 RSC.
- S. Liu, J. Tian, L. Wang, Y. Zhang, X. Qin, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Adv. Mater., 2012, 24, 2037–2041 CrossRef CAS PubMed.
- M. Algarra, B. B. Campos, K. Radotić, D. Mutavdžić, T. Bandosz, J. Jiménez-Jiménez, E. Rodriguez-Castellón and J. C. G. Esteves da Silva, J. Mater. Chem. A, 2014, 2, 8342 CAS.
- Y. Liu, Y. Liu, S. J. Park, Y. Zhang, T. Kim, S. Chae, M. Park and H. Y. Kim, J. Mater. Chem. A, 2015, 3, 17747–17754 CAS.
- W. J. Bai, H. Z. Zheng, Y. J. Long, X. J. Mao, M. Gao and L. Y. Zhang, Anal. Sci., 2011, 27, 243–246 CrossRef CAS PubMed.
- J. Wang, J. Wei, S. Su and J. Qiu, New J. Chem., 2015, 39, 501–507 RSC.
- K. Qu, J. Wang, J. Ren and X. Qu, Chem.–Eur. J., 2013, 19, 7243–7249 CrossRef CAS PubMed.
- P. Shen and Y. Xia, Anal. Chem., 2014, 86, 5323–5329 CrossRef CAS PubMed.
- P. M. Carrasco, I. García, L. Yate, R. Tena Zaera, G. Cabañero, H. J. Grande and V. Ruiz, Carbon, 2016, 109, 658–665 CrossRef.
- T. Tian, Y. He, Y. Ge and G. Song, Sens. Actuators, B, 2017, 240, 1265–1271 CrossRef CAS.
- H. Y. Zhang, Y. Wang, S. Xiao, H. Wang, J. H. Wang and L. Feng, Biosens. Bioelectron., 2016, 87, 46–52 CrossRef PubMed.
- L. Bu, J. Peng, H. Peng, S. Liu, H. Xiao, D. Liu, Z. Pan, Y. Chen, F. Chen and Y. He, RSC Adv., 2016, 6, 95469–95475 RSC.
- P. C. Hsu, P. C. Chen, C. M. Ou, H. Y. Chang and H. T. Chang, J. Mater. Chem. B, 2013, 1, 1774–1781 RSC.
- L. Wang and H. S. Zhou, Anal. Chem., 2014, 86, 8902–8905 CrossRef CAS PubMed.
- S. J. Zhao, M. H. Lan, X. Y. Zhu, H. T. Xue, T. W. Ng, X. M. Meng, C. S. Lee, P. F. Wang and W. J. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 17054–17060 CAS.
- W. Li, Z. Yue, C. Wang, W. Zhang and G. Liu, RSC Adv., 2013, 3, 20662 RSC.
- J. Zhou, Z. Sheng, H. Han, M. Zou and C. Li, Mater. Lett., 2012, 66, 222–224 CrossRef CAS.
- X. M. Yang, Y. Zhuo, S. S. Zhu, Y. W. Luo, Y. J. Feng and Y. Dou, Biosens. Bioelectron., 2014, 60, 292–298 CrossRef CAS PubMed.
- D. Sun, R. Ban, P.-H. Zhang, G.-H. Wu, J.-R. Zhang and J.-J. Zhu, Carbon, 2013, 64, 424–434 CrossRef CAS.
- L. Li, G. Wu, T. Hong, Z. Yin, D. Sun, E. S. Abdel-Halim and J. J. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 2858–2864 CAS.
- Y. Lei, F. Chen, Y. Luo and L. Zhang, J. Mater. Sci., 2014, 49, 4236–4245 CrossRef CAS.
- L. Zhang, C. Xu and B. Li, Microchim. Acta, 2009, 166, 61–68 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21059g |
| This journal is © The Royal Society of Chemistry 2016 |