Novel synthetic strategy towards NiO/Ni3N composite hollow nanofibers for superior NOx gas-sensing properties at room temperature
Abstract
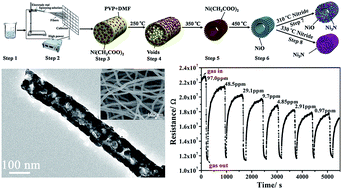
NiO hollow nanofibers were successfully synthesized via a facile electrospinning followed by post-calcination process, NiO/Ni3N composite hollow nanofibers and Ni3N nanofibers were prepared by nitridation of the prepared NiO hollow nanofibers in the presence of NH3 atmosphere at 310 °C and 330 °C, respectively. The crystal structure, morphology and compositions of NiO hollow nanofibers, NiO/Ni3N composite hollow nanofibers and Ni3N nanofibers were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive spectrometry (EDS). Possible formation mechanism of samples was also proposed. By using NOx as a probe molecule, compared with NiO hollow nanofibers and Ni3N nanofibers, NiO/Ni3N composite hollow nanofibers exhibit more excellent sensing performances in terms of high response, fast response time, nice selectivity and excellent stability at room temperature. The outstanding performances in gas sensing of NiO/Ni3N composite hollow nanofibers owe to one-dimensional hollow fibrous nanostructure and the unique chemical composition, and NiO/Ni3N composite hollow nanofibers are promising material for NOx gas sensor at room temperature.

 Please wait while we load your content...
Please wait while we load your content...