Carbon dots–quinoline derivative nanocomposite: facile synthesis and application as a “turn-off” fluorescent chemosensor for detection of Cu2+ ions in tap water†
Qian Lia,
Qitong Huangab,
Jie-Ji Zhua,
Wen-Gang Jia and
Qing-Xiao Tong*a
aDepartment of Chemistry, Shantou University, Guangdong 515063, P. R. China. E-mail: qxtong@stu.edu.cn; Fax: +86 754 86500648; Tel: +86 754 86502508
bDepartment of Food and Biological Engineering, Zhangzhou Institute of Technology, Zhangzhou, 363000, China
First published on 7th September 2016
Abstract
In this paper, a novel quinoline derivative: 8-(pyridin-2-ylmethoxy)quinoline-2-carboxylic acid (Q) has been successfully synthesized, then it was coupled with carbon dots (CDs) to synthesize a CDs–Q nanocomposite by a simple and green method. The CDs–Q nanocomposite can act as a “turn-off” fluorescent chemosensor for detection of Cu2+ ions. It's a good candidate for Cu2+ sensing on account of its excellent properties such as its high sensitivity and selectivity, easy synthesis, excellent fluorescence stability towards temperature and pH, direct detection in aqueous solution and tap water and distinct fluorescence quenching towards Cu2+. We have also discussed the fluorescence quenching mechanism of CDs–Q and the possible coordination mode of CDs–Q with Cu2+. CDs–Q have a wide linear range (0.01–1.2 μM) and high sensitivity towards Cu2+ ions with a detection limit of 0.56 nM. In addition, this fluorescent chemosensor was also applied to determine Cu2+ in tap water with satisfying results.
1. Introduction
Copper, the third abundant transition-metal in the human body, plays a crucial role in maintaining and adjusting the metabolism of the human body. Copper-containing enzymes such as superoxide dismutase (SOD) could catalyze some fundamental cellular processes. In addition, it will affect physiological processes such as bone formation and cellular respiration.1–4 Once the concentration of copper ions is unbalanced in the body, it can bring about a variety of diseases and problems, including Alzheimer's disease, prion diseases, familial diseases such as muscular dystrophy and Parkinson's disease, gastrointestinal disorders and liver or kidney damage.5 On the other hand, when Cu2+ gathers to a certain amount it is toxic to environment.6 Studies on Cu2+ have great significance in the field of biological and environmental science. Therefore, it is imperative to develop highly sensitive and selective sensors for efficient detection of Cu2+.Traditional analytical techniques for Cu2+, which includes atomic absorption spectroscopy,7 inductively coupled plasma mass spectrometry,8 inductively coupled plasma atomic emission spectrometry,9 high-resolution continuum source atomic absorption spectrometry10 are expensive and require extensive sample preparation and pretreatment. Electrochemical detection of Cu2+ is simple and fast, but the output signal is generally unstable.11–13 Compared to the above technology, fluorescent sensors have some unique properties such as high selectivity, operational simplicity, amenable miniaturization and real-time detection.14–17 Therefore, a series of fluorescent sensors of Cu2+ have been designed and developed. Up to now, many Cu2+ fluorescent sensors have been reported, while most of them are based on small organic fluorophores, very few are based on fluorescent nanocomposites. Considering that both of them suffered from some limitations, their practical application is relatively limited. For instance, sensors based on small organic molecules exhibited unstable photochemical properties, high toxicity, poor biocompatibility.18 Furthermore, they usually detect in pure organic solvent or solvents containing very little aqueous solution and need strict operation condition. Sensors based on fluorescent nanocomposite show poor selectivity in the presence of other metal ions. Therefore, it is still a difficult and challenging task to design and synthesize a novel Cu2+ fluorescent sensor which has high selectivity and sensitivity and can directly detect in aqueous solution. To achieve this goal, we imagine developing a fluorescent sensor that can combine the above two to make best use of the advantages and bypass the disadvantages.
8-hydroxyquinoline19–21 has attracted widespread attention recently due to its peculiar properties which can be used as both fluorophore and receptor unit. But researchers usually modify or introduce auxiliary ligand groups in the quinoline ring to improve selectivity because of its poor selectivity to various metal ions. CDs, as one of new members of the carbon nanomaterials, has many advantages such as good photostability, excellent solubility in aqueous solution, low biological toxicity, good chemical stability, non-blinking and easiness to functionalize.22–28 Thus, CDs have received considerable attention owing to their outstanding properties. A growing number of fluorescent sensors based on CDs have been reported, but some suffer from poor selectivity,29 low sensitivity17,30 or narrow linear range.31,32 Hence, we envisage to synthesize a sensor (CDs–Q) which integrates the advantages of both CDs and 8-hydroxyquinoline derivatives. In this paper, we have investigated systematically the photochemical and photophysical properties and response performance of CDs–Q. To the best of our knowledge, it is a unique receptor showing high sensitivity towards Cu2+ ions with a detection limit of 0.56 nM. More importantly, CDs–Q can be applied to detect Cu2+ in tap water.
2. Experimental detail
2.1 Reagents and instrumentation
The metal salts (FeSO4·7H2O, FeCl3·6H2O, CuSO4·5H2O, HgCl2, Pb(NO3)2, CaCl2, ZnSO4·7H2O, MgSO4, NiSO4·6H2O, CoCl2·6H2O, KCl, NaCl) were purchased from aladdin. Unless particularly noted, all reagents were purchased and used without further purification. 1H NMR spectra and 13C NMR were recorded on a Bruker Avance 400 spectrometer (400 MHz) using TMS as internal standard. Absorption and photoluminescence spectra were determined with a Perkin-Elmer Lambda UV-Vis-2600 spectrophotometer and a Perkin-Elmer LS50B Luminescence spectrophotometer. Fluorescence lifetimes were recorded with PTI QM-TM. Infrared spectra were recorded with a Nicolet Avatar 360 FTIR spectrometer in the range of 4000–400 cm−1 (KBr disk). TEM images were carried out on a JEM-100CX electron microscope (JEOL Ltd, Aichi Kariya, Japan).2.2 Synthesis of Q
A synthetic route for compound Q was depicted in Scheme 1 (1H NMR, 13C NMR and MS showed in Fig. S1–S6†). Compound 1 were synthesized according to the literature method.33,34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). After that, NaClO2 (1.55 g, 12.8 mmol) and H2NSO3H (1.16 g, 12.8 mmol) was added into the above mixed solution in an ice bath. After stirring for 30 min at room temperature, THF was evaporated under reduced pressure. Then the remainder mixture was filtered to remove remaining water. The filter cake was light yellow solid (compound 2). Yield: 0.78 g (87%).
1, v/v). After that, NaClO2 (1.55 g, 12.8 mmol) and H2NSO3H (1.16 g, 12.8 mmol) was added into the above mixed solution in an ice bath. After stirring for 30 min at room temperature, THF was evaporated under reduced pressure. Then the remainder mixture was filtered to remove remaining water. The filter cake was light yellow solid (compound 2). Yield: 0.78 g (87%).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to afford compound 3 as a white solid. Yield: 0.38 g (61%). 1H NMR (400 MHz, DMSO-d6, TMS) δH ppm: 8.70 (dd, J = 8.8, 2.3 Hz, 1H), 8.61 (d, J = 4.7 Hz, 1H), 8.29 (dd, J = 8.8, 1.7 Hz, 1H), 7.93–7.77 (m, 2H), 7.72 (d, J = 7.8 Hz, 1H), 7.38 (t, J = 6.5 Hz, 2H), 5.49 (s, 2H), 4.45 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CD2Cl2): δ 165.49, 156.88, 154.92, 148.82, 148.51, 141.13, 138.36, 134.68, 129.00, 128.74, 123.66, 123.41, 122.80, 122.69, 111.94, 72.28, 62.58, 14.63.
1, v/v) as eluent to afford compound 3 as a white solid. Yield: 0.38 g (61%). 1H NMR (400 MHz, DMSO-d6, TMS) δH ppm: 8.70 (dd, J = 8.8, 2.3 Hz, 1H), 8.61 (d, J = 4.7 Hz, 1H), 8.29 (dd, J = 8.8, 1.7 Hz, 1H), 7.93–7.77 (m, 2H), 7.72 (d, J = 7.8 Hz, 1H), 7.38 (t, J = 6.5 Hz, 2H), 5.49 (s, 2H), 4.45 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CD2Cl2): δ 165.49, 156.88, 154.92, 148.82, 148.51, 141.13, 138.36, 134.68, 129.00, 128.74, 123.66, 123.41, 122.80, 122.69, 111.94, 72.28, 62.58, 14.63.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to obtain Q as a white solid. Yield: 0.26 g (90%). 1H NMR (400 MHz, DMSO-d6, TMS) δH ppm: 9.77 (s, 1H), 8.70 (d, J = 8.7 Hz, 1H), 8.63 (d, J = 4.7 Hz, 1H), 8.26 (d, J = 8.7 Hz, 1H), 7.87 (td, J = 7.7, 1.6 Hz, 1H), 7.78 (d, J = 8.5 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.44–7.31 (m, 2H), 5.76 (s, 1H), 5.49 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO): δ 162.51, 156.24, 153.45, 149.35, 148.95, 138.91, 136.88, 134.11, 128.01, 127.03, 122.88, 121.72, 121.45, 120.09, 112.23, 71.85.
1, v/v) as eluent to obtain Q as a white solid. Yield: 0.26 g (90%). 1H NMR (400 MHz, DMSO-d6, TMS) δH ppm: 9.77 (s, 1H), 8.70 (d, J = 8.7 Hz, 1H), 8.63 (d, J = 4.7 Hz, 1H), 8.26 (d, J = 8.7 Hz, 1H), 7.87 (td, J = 7.7, 1.6 Hz, 1H), 7.78 (d, J = 8.5 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.44–7.31 (m, 2H), 5.76 (s, 1H), 5.49 (s, 2H), 4.74 (s, 2H). 13C NMR (101 MHz, DMSO): δ 162.51, 156.24, 153.45, 149.35, 148.95, 138.91, 136.88, 134.11, 128.01, 127.03, 122.88, 121.72, 121.45, 120.09, 112.23, 71.85.2.3 Preparation of Q modified-CDs
CDs were synthesized by previously reported method.35–37 For the preparation of CDs–Q: 10.00 mL, 10.00 mM EDC and 5.00 mL, 10.00 mM NHS were added to 10.00 mL 8.00 mg mL−1 CDs solution and incubated at 37 °C for 30 min. Then 10.00 mL, 10.00 mg mL−1 Q solution of MeOH was added to the reaction mixture and further activated at 37 °C for 2 h. Then, the solution was kept at 4 °C overnight to deactivate the remaining EDC and NHS. The conjugate solution was centrifugated at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and then the supernate was dialyzed overnight with dialysis membranes of 5000 cutoffs to separate excess CDs, Q, EDC and NHS. Finally, the supernate were freeze-dried to give CDs–Q as a yellow solid. Yield: 167.0 mg.
000 rpm for 30 min and then the supernate was dialyzed overnight with dialysis membranes of 5000 cutoffs to separate excess CDs, Q, EDC and NHS. Finally, the supernate were freeze-dried to give CDs–Q as a yellow solid. Yield: 167.0 mg.
2.4 Fluorescent detection of Cu2+
2.00 mL 0.80 mg mL−1 CDs–Q were placed in a cuvette. Following this, 100 μL various metal ions of different concentrations was added. Among them, 10 μL Cu2+ of different concentrations were increasingly added in linear range experiments. Make sure that the volume of metal ions is less than 5% of the total volume. Simultaneously, a reagent blank experiment was carried out. Finally, fluorescence intensity of the detection solution (F) and the blank solution (F0) were recorded and F/F0 was calculated.2.5 Quantum yield measurement
The quantum yield (Φ) were measured by comparing the integrated photoluminescence intensities and the absorbency values of the CDs, Q or CDs–Q with the reference quinine sulfate. The quinine sulfate (literature ΦR = 0.54) was dissolved in 0.1 M H2SO4.| Φ = ΦR (I/IR) (AR/A) (η/ηR)2 |
3. Results and discussion
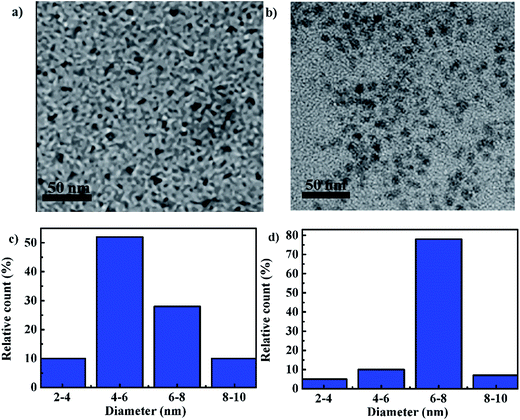
Both CDs and CDs–Q were well distributed, monodisperse and approximate spherical in shape under transmission electron microscope, as shown in Fig. 1. The average diameter of CDs was ca. 5 nm. After modified with Q, the corresponding average diameter of CDs–Q increased to ca. 7 nm, implying successful coupling interaction between Q and CDs.To further ascertain that Q have been reacted with CDs, we studied the spectral properties of CDs, CDs–Q and Q, as shown in Fig. 2. Fig. 2 showed that there are significant differences in UV-vis absorption spectra and fluorescence spectra of CDs, CDs–Q and Q. As shown in Fig. 2a, the maximum absorption wavelength of CDs, CDs–Q and Q were at 282, 231 and 233 nm, respectively. These peak can be assigned to the π–π* transitions of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in sp2 hybrid domains. In UV-vis absorption spectra of CDs–Q, there also existed a broad absorption band from 260 to 330 nm, which was ascribed to the n–π* transition of C
C in sp2 hybrid domains. In UV-vis absorption spectra of CDs–Q, there also existed a broad absorption band from 260 to 330 nm, which was ascribed to the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N or C–O in sp3 hybrid regions. Additionally, compared with Q alone, this was a new optical absorption peak. Thus, we deduced that coupling reaction produced new amide bond which further lead to a broad absorption band in this wavelength range. We also performed the UV-vis titration of CDs, CD–Q and Q alone to assess the binding of Cu(II) to either, as shown in Fig. S7.† We found that the absorbance of CDs was almost unchanged and the absorbance of Q reduced slightly in the presence of Cu2+. While the absorbance of CDs–Q decreased greatly at equal concentrations of Cu2+. This indicated that the formation of CDs–Q raised response performance. As shown in Fig. S7d,† when the concentration of Cu2+ was 1.5 μM, the UV-vis absorption spectra was almost the same as the 1.2 μM Cu2+ in the 2.00 mL 0.80 mg mL−1 CDs–Q. Hence, 1.2 μM Cu2+ was the largest concentration reacted with 2.00 mL 0.80 mg mL−1 CDs–Q. The maximum emission wavelength of CDs, CDs–Q and Q were at 448, 464 and 481 nm, respectively, which were shown in Fig. 2b. The new material has its own luminescence properties. And the UV-vis and TEM both showed the CDs–Q was a new material. The fluorescence quantum yield of CDs, CDs–Q and Q were calculated to be 5.3%, 22.6% and 11.7%, respectively. Compared to CDs and Q, the QY of CDs–Q increased nearly 3 times (CDs) and 1 time (Q), respectively. Q was almost completely insoluble in aqueous solution and most organic solvents such as CH2Cl2, CHCl3 and CH3CN. After reacting with CDs to generate CDs–Q, water dispersibility is greatly raising.
O, C–N or C–O in sp3 hybrid regions. Additionally, compared with Q alone, this was a new optical absorption peak. Thus, we deduced that coupling reaction produced new amide bond which further lead to a broad absorption band in this wavelength range. We also performed the UV-vis titration of CDs, CD–Q and Q alone to assess the binding of Cu(II) to either, as shown in Fig. S7.† We found that the absorbance of CDs was almost unchanged and the absorbance of Q reduced slightly in the presence of Cu2+. While the absorbance of CDs–Q decreased greatly at equal concentrations of Cu2+. This indicated that the formation of CDs–Q raised response performance. As shown in Fig. S7d,† when the concentration of Cu2+ was 1.5 μM, the UV-vis absorption spectra was almost the same as the 1.2 μM Cu2+ in the 2.00 mL 0.80 mg mL−1 CDs–Q. Hence, 1.2 μM Cu2+ was the largest concentration reacted with 2.00 mL 0.80 mg mL−1 CDs–Q. The maximum emission wavelength of CDs, CDs–Q and Q were at 448, 464 and 481 nm, respectively, which were shown in Fig. 2b. The new material has its own luminescence properties. And the UV-vis and TEM both showed the CDs–Q was a new material. The fluorescence quantum yield of CDs, CDs–Q and Q were calculated to be 5.3%, 22.6% and 11.7%, respectively. Compared to CDs and Q, the QY of CDs–Q increased nearly 3 times (CDs) and 1 time (Q), respectively. Q was almost completely insoluble in aqueous solution and most organic solvents such as CH2Cl2, CHCl3 and CH3CN. After reacting with CDs to generate CDs–Q, water dispersibility is greatly raising.
 | ||
| Fig. 2 UV-vis absorption spectra (a) and fluorescence spectra (b) of 2.00 mL 0.80 mg mL−1 CDs, CDs–Q and Q (condition: CDs (λex = 366 nm), CDs–Q (λex = 345 nm) and Q (λex = 345 nm)). | ||
FTIR spectra of CDs–Q are different from CDs and Q, as shown in Fig. 3. In IR spectroscopy of Q, the narrow bands at around 3404 and 1680 cm−1 correspond to the stretching vibration of –NH2 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(–CONH–), respectively. In IR spectroscopy of CDs, the broad bands at around 3401 and 1642 cm−1 demonstrate the existence of –OH and C
O(–CONH–), respectively. In IR spectroscopy of CDs, the broad bands at around 3401 and 1642 cm−1 demonstrate the existence of –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. The other bands were attributed as follows: 2915 cm−1 for C–H stretching vibration and 1101 cm−1 for C–O stretching, respectively. Compared with CDs and Q, absorption bands of –NH2 disappears and two new absorption bands appear between 1500 and 1700 cm−1 in IR spectroscopy of CDs–Q. The band at 3370 cm−1 represents stretching vibration of –OH. 1648 and 1565 cm−1 were assigned to stretching vibration of N–C
O, respectively. The other bands were attributed as follows: 2915 cm−1 for C–H stretching vibration and 1101 cm−1 for C–O stretching, respectively. Compared with CDs and Q, absorption bands of –NH2 disappears and two new absorption bands appear between 1500 and 1700 cm−1 in IR spectroscopy of CDs–Q. The band at 3370 cm−1 represents stretching vibration of –OH. 1648 and 1565 cm−1 were assigned to stretching vibration of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.38 All above is well in line with the results of TEM.
O.38 All above is well in line with the results of TEM.
 | ||
| Fig. 3 FTIR spectra of CDs, CDs–Q and Q (note: aqueous solution of CDs and CDs–Q were dried in a vacuum oven at 60 °C for 48 hours). | ||
As shown in Fig. 4a, the fluorescence intensity of CDs–Q was drastically reduced upon the addition of Cu2+, which indicated it was a “turn-off” sensor. The uncertainty of specific coupling ratio of CDs and Q leads to uncertain Q content in CDs–Q, this further bring to new problem for mechanism study. We can't obtain the specific coordination mode by Job's plot curve. Although the real mode of action was still unknown at present, we attentively assumed that the possible reason of fluorescence quenching maybe induced by multi-site coordination of CDs–Q (N^O^N^N) with Cu2+, as shown in Scheme 2. Afterwards, it maybe generate a stable nonluminous ground state complex, which further lead to the fluorescence quenching. To understand the mechanism of the fluorescence quenching of CDs–Q by Cu2+, we measured the fluorescence lifetime of CDs–Q and CDs–Q/Cu2+, as shown in Fig. 4b. CDs–Q and CDs–Q/Cu2+ both fit single exponential function with lifetimes of 20.10 ns and 19.96 ns, respectively. The slightly reduced lifetimes indicated that a static quenching occurs because of the formation of a nonluminous ground state complex between CDs–Q and Cu2+. Fig. 4b also showed the fluorescence lifetime of CDs and Q, which were 3.26 and 2.77 ns, respectively. These data showed that the as-prepared CDs–Q significantly improved the fluorescence lifetime compared to CDs and Q.
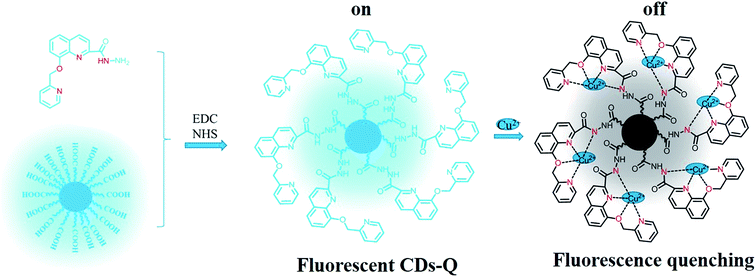 | ||
| Scheme 2 A schematic illustration of the synthesis of CDs–Q and proposed coordination mode of CDs–Q with Cu2+. | ||
The influence of temperature and pH on the fluorescence intensity were also investigated. As shown in Fig. S8a,† with the change of temperature, the fluorescence intensity reached a minimum at 25 °C in the range of 20–70 °C. With the pH range from 2 to 12, the fluorescence intensity reached a minimum at pH 7.0, as shown in Fig. S8b.† When the pH values increased from 2.0 to 7.0, the fluorescence intensity was decreased. Nevertheless, when the pH was beyond 7.0, the fluorescence intensity was conversely increased. According to Fig. 5, the general trend of the intensity is essentially stable with pH range from 3.5 to 11.5 and temperature from 20 to 70 °C. These research observations indicate that CDs-based fluorescent sensor is more stable against temperature and pH variation than some organic fluorophores.39,40
 | ||
| Fig. 5 Influence of temperature (a) and pH (b) on the fluorescence intensity of the system at 464 nm. (Conditions: 2.00 mL 0.80 mg mL−1 CDs–Q, CCu2+ = 5 × 10−7 M.) | ||
Selectivity and sensitivity are the important index for an excellent fluorescent sensor. Therefore, we observed the fluorescent response of CDs–Q in the presence of other metal cations at room temperature. Meanwhile, we gained the fluorescence intensity in the case of various concentration of copper ions and obtained corresponding working curve, linear range and limit of detection (LOD). The interference experiments were investigated by monitoring the response of CDs–Q at 464 nm to other possible coexisting metal cations under the same conditions, as shown in Fig. 6. It was observed that most ions (Mg2+, Fe2+, Ni2+, Ca2+, K+, Na+, Zn2+, Pb2+, Hg2+, Fe3+, Co2+) induced negligible interfering effects, while Hg2+ caused a little fluorescence decrease. Only Cu2+ can lead to appreciable quenching of fluorescence of the system. These results attested the selectivity of the as-prepared CDs–Q for Cu2+ detection.
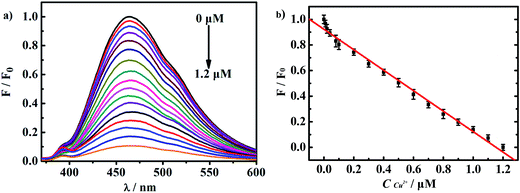
The working curve for Cu2+ detection was carried out by recording the fluorescence response when adding various concentration of copper ions, as shown in Fig. 7. With the amount of copper ions gradually increasing, the fluorescence was gradually quenched. The fluorescence almost completely quenched when the concentration of Cu2+ reached 1.2 μM. F/F0 showed a good linear relationship with CCu2+ in a wide range which covered from 0.01 to 1.2 μM. The linear fitting equation was F/F0 = 0.9311–0.7144 CCu2+ (μM) with a high correlation coefficient of 0.9950. The LOD was as low as 0.56 nM. Then we compared the LOD and linear range of several different CDs for Cu2+ detection, as shown in Table 1. We discovered that linear range was wide enough and the LOD was quite lower than some sensors based on CDs.17,28,31,32 These data indicates the excellent sensitivity of this sensor for detecting copper content.
Finally, to explore the reliability of this fluorescent chemosensor, we carried out a standard recovery experiment in linear range, as shown in Table 2. The recovery in tap water sample is 102.5–105.5% that is in the error-permitted range. The experimental results proved that the sensor can be applied to actually detect the content of copper ions in tap water.
| Tap water | Spiked (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Sample 1 | 0.100 | 0.103 | 103.0 | 1.6 |
| Sample 2 | 0.200 | 0.205 | 102.5 | 2.2 |
| Sample 3 | 0.300 | 0.312 | 104.0 | 1.5 |
| Sample 4 | 0.400 | 0.422 | 105.5 | 3.1 |
4. Conclusions
As expected, we have successfully synthesized a unique receptor Q that has poor solubility in aqueous solution. Then Q coupled with CDs to develop CDs–Q, which could significantly increase the water solubility and fluorescence intensity. CDs–Q integrate good qualities of CDs and Q for detection of Cu2+, such as easy synthesis, good selectivity, high sensitivity, wide linear range (0.01–1.2 μM), low detection limit (0.56 nM), excellent fluorescence stability towards temperature and pH. At the same time, CDs–Q can act as a good candidate to be applied to detect Cu2+ in tap water.Acknowledgements
This research was supported by the National Basic Research Program of China (973 Program No. 2013CB834803), the National Natural Science Foundation of China (Project No. 51273108), the Education Bureau of Fujian Province (Project No. JAT160875), the Natural Science Foundation of Zhangzhou (Project No. ZZ2016J31) and the Training Program Foundation for Excellent Youth Researching Talents of Fujian's Universities (Fujian Education Section, 2016, No. 23).References
- J. A. Cotruvo Jr, A. T. Aron, K. M. Ramos-Torres and C. J. Chang, Chem. Soc. Rev., 2015, 44, 4400–4414 RSC.
- T. Wu, T. Xu and Z. Ma, Analyst, 2015, 140, 8041–8047 RSC.
- S. X. Li, X. Lin, F. Y. Zheng, W. Liang, Y. Zhong and J. Cai, Anal. Chem., 2014, 86, 7079–7083 CrossRef CAS PubMed.
- D. E. Kang, C. S. Lim, J. Y. Kim, E. S. Kim, H. J. Chun and B. R. Cho, Anal. Chem., 2014, 86, 5353–5359 CrossRef CAS PubMed.
- C. Vulpe, B. Levinson, S. Whitney, S. Packman and J. Gitschier, Nat. Genet., 1993, 3, 7–13 CrossRef CAS PubMed.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995–2004 CrossRef CAS PubMed.
- Y. E. Unsal, M. Soylak, M. Tuzen and B. Hazer, Anal. Lett., 2015, 48, 1163–1179 CrossRef CAS.
- H. Wang, Z. Wu, B. Chen, M. He and B. Hu, Analyst, 2015, 140, 5619–5626 RSC.
- V. Shkirskiy, A. D. King, O. Gharbi, P. Volovitch, J. R. Scully, K. Ogle and N. Birbilis, ChemPhysChem, 2015, 16, 536–539 CrossRef CAS PubMed.
- A. S. N. Trindade, A. F. Dantas, D. C. Lima, S. L. C. Ferreira and L. S. Teixeira, Food Chem., 2015, 185, 145–150 CrossRef CAS PubMed.
- F. Yang, D. He, B. Zheng, D. Xiao, L. Wu and Y. Guo, J. Electroanal. Chem., 2016, 767, 100–107 CrossRef CAS.
- X. Gan, H. Zhao, X. Quan and Y. Zhang, Electrochim. Acta, 2016, 190, 480–489 CrossRef CAS.
- J. Li, L. Zhang, G. Wei, Y. Zhang and Y. Zeng, Biosens. Bioelectron., 2015, 69, 316–320 CrossRef CAS PubMed.
- Y. Song, D. Hu, F. Liu, S. Chen and L. Wang, Analyst, 2015, 140, 623–629 RSC.
- X. Sun, P. Liu, L. Wu and B. Liu, Analyst, 2015, 140, 6742–6747 RSC.
- B. Muthuraj, S. R. Chowdhury, S. Mukherjee, C. R. Patra and P. K. Iyer, RSC Adv., 2015, 5, 28211–28218 RSC.
- X. Liu, N. Zhang, T. Bing and D. Shangguan, Anal. Chem., 2014, 86, 2289–2296 CrossRef CAS PubMed.
- H. Mattoussi, J. M. Mauro, E. R. Goldman, G. P. Anderson, V. C. Sundar, F. V. Mikulec and M. G. Bawendi, J. Am. Chem. Soc., 2000, 122, 12142–12150 CrossRef CAS.
- C. Liu, C. Shi, H. Li, W. Du, Z. Li, L. Wei and M. Yu, Sens. Actuators, B, 2015, 219, 185–191 CrossRef CAS.
- H. Zhang, L. F. Han, K. A. Zachariasse and Y. B. Jiang, Org. Lett., 2005, 7, 4217–4220 CrossRef CAS PubMed.
- M. Mameli, M. C. Aragoni, M. Arca, C. Caltagirone, F. Demartin, G. Farruggia and V. Lippolis, Chem.–Eur. J., 2010, 16, 919–930 CrossRef CAS PubMed.
- Y. Du and S. Guo, Nanoscale, 2016, 8, 2532–2543 RSC.
- Q. Huang, X. Lin, F. Li, W. Weng, L. Lin and S. Hu, Progr. Chem., 2015, 27, 1604–1614 Search PubMed.
- R. Purbia and S. Paria, Biosens. Bioelectron., 2016, 79, 467–475 CrossRef CAS PubMed.
- J. B. Essner, C. H. Laber, S. Ravula, L. Polo-Parada and G. A. Baker, Green Chem., 2016, 18, 243–250 RSC.
- B. B. Chen, Z. X. Liu, H. Y. Zou and C. Z. Huang, Analyst, 2016, 141, 2676–2681 RSC.
- Q. Huang, X. Lin, C. Lin, Y. Zhang, S. Hu and C. Wei, RSC Adv., 2015, 5, 54102–54108 RSC.
- J. Xia, J. Di, H. Li, H. Xu, H. Li and S. Guo, Appl. Catal., B, 2016, 181, 260–269 CrossRef CAS.
- G. Gedda, C. Y. Lee, Y. C. Lin and H. F. Wu, Sens. Actuators, B, 2016, 224, 396–403 CrossRef CAS.
- A. Zhu, Q. Qu, X. Shao, B. Kong and Y. Tian, Angew. Chem., 2012, 124, 7297–7301 CrossRef.
- J. Zong, X. Yang, A. Trinchi, S. Hardin, I. Cole, Y. Zhu and G. Wei, Biosens. Bioelectron., 2014, 51, 330–335 CrossRef CAS PubMed.
- Y. Dong, R. Wang, G. Li, C. Chen, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 6220–6224 CrossRef CAS PubMed.
- E. Terazzi, L. Guenee, B. Bocquet, J. F. Lemonnier, N. Dalla Favera and C. Piguet, Chem.–Eur. J., 2009, 15, 12719–12732 CrossRef CAS PubMed.
- L. Xue, H. H. Wang, X. J. Wang and H. Jiang, Inorg. Chem., 2008, 47, 4310–4318 CrossRef CAS PubMed.
- Q. Huang, S. Hu, H. Zhang, J. Chen, Y. He, F. Li, W. Weng, J. Ni, X. Bao and Y. Lin, Analyst, 2013, 138, 5417–5423 RSC.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118–5120 RSC.
- J. M. Liu, L. P. Lin, X. X. Wang, L. Jiao, M. L. Cui, S. L. Jiang, W. L. Cai, L. H. Zhang and Z. Y. Zheng, Analyst, 2013, 138, 278–283 RSC.
- X. Cui, L. Zhu, J. Wu, Y. Hou, P. Wang, Z. Wang and M. Yang, Biosens. Bioelectron., 2015, 63, 506–512 CrossRef CAS PubMed.
- T. Hirata, T. Terai, H. Yamamura, M. Shimonishi, T. Komatsu, K. Hanaoka, T. Ueno, Y. Imaizumi, T. Nagano and Y. Urano, Anal. Chem., 2016, 88, 2693–2700 CrossRef CAS PubMed.
- J. Cui, D. P. Li, S. L. Shen, J. T. Liu and B. X. Zhao, RSC Adv., 2015, 5, 3875–3880 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21034a |
| This journal is © The Royal Society of Chemistry 2016 |