Fabrication of highly visible-light-responsive ZnFe2O4/TiO2 heterostructures for the enhanced photocatalytic degradation of organic dyes†
Thanh Binh Nguyena and
Ruey-an Doong *ab
*ab
aDepartment of Biomedical Engineering and Environmental Sciences, National Tsing Hua University, Hsinchu, 30013, Taiwan
bInstitute of Environmental Engineering, National Chiao Tung University, Hsinchu, 30010, Taiwan. E-mail: radoong@nctu.edu.tw
First published on 17th October 2016
Abstract
In this study, a novel visible-light-sensitive ZnFe2O4–TiO2 photocatalyst has been fabricated by coupling 0.2–2 wt% p-type ZnFe2O4 narrow bandgap material with n-type anatase TiO2 for the enhanced photocatalytic degradation of organic dyes under 465 nm visible light irradiation. Transmission electron microscopy (TEM) and high resolution TEM confirm that ZnFe2O4 and TiO2 are strongly linked with an average particle size of 8–9 nm, leading to a decrease in hole–electron recombination rate as well as the enhanced photocatalytic activity of the ZnFe2O4–TiO2 heterostructures under visible light irradiation. The optimized 1 wt% ZnFe2O4 not only significantly extends the absorption edge of TiO2-based heterostructures to the visible light region but can also retain a stable photodegradation efficiency of >99% for at least 5 cycles. In addition, the photocatalytic activity of ZnFe2O4–TiO2 toward dye decomposition follows the order cationic rhodamine B > neutral methyl red > anionic methyl orange. Our results clearly demonstrate that the coupling of a low loading mass of ZnFe2O4 with anatase TiO2 is a reliable green technology approach to prepare visible-light-responsive heterostructure photocatalysts with great potential for application in the decomposition of organic dyes and other emerging pollutants in the treatment of water and wastewater.
1. Introduction
The search for and development of excellent semiconductor photocatalysts such as titanium dioxide (TiO2), zinc oxide (ZnO) and tungsten oxide (WO3) for water purification and wastewater treatment has recently attracted tremendous attention.1 Among the photocatalytic materials used, TiO2 nanoparticles are regarded as excellent photocatalysts because of their high photoreactivity, good photostability, non-toxicity and environmental friendliness.2 Nevertheless, TiO2 has a wide bandgap of 3.0–3.2 eV and the implementation of pure TiO2 in practical photocatalytic applications is still seriously limited in the visible light region, which occupies around 45% of the total sunlight spectrum.3 Another limitation of TiO2 photocatalysts is the rapid recombination of electrons and holes. Therefore, it is urgently needed to develop a novel visible-light-responsive TiO2 based photocatalyst for environmental applications.Several methods including doping and surface modification have been attempted to extend the absorption wavelength of TiO2 to the visible light region.4–9 Several metal and non-metal ions such as Cu, Ag, Au, Fe, N, C, S and F in the concentration range of 0.5–10 wt% have been added as dopants for the fabrication of visible-light-driven TiO2 based photocatalysts for the effective degradation of organic pollutants and dyes.10–17 In addition, the combination of TiO2 with other narrow bandgap semiconductors such as ZnS, RuO2, CdS and WO3 has emerged as an important strategy to reduce the bandgap by introducing energy levels between the conduction (CB) and valence bands (VB),18–21 thus allowing TiO2 to be active under visible light irradiation. Over the past decades, heterostructures such as ZnO/TiO2,22,23 CdS/TiO2 (ref. 24 and 25) and CuO/TiO2 nanorods26,27 have been developed for the degradation of organic pollutants and water splitting under UV-visible light irradiation. Such nanocomposite structures also favor the enhancement of photoinduced electron–hole separation, and subsequently lead to an acceleration in the efficiency and rate of pollutant photodegradation.
Of particular interest in the development of visible-light-responsive photocatalysts is the synthesis of nanocrystalline spinel ferrite. Several ferrites including NiFe2O4, ZnFe2O4 and CuFe2O4 have been reported to possess excellent photocatalytic activity for the removal of inorganic contaminants,28 antimicrobial activity29,30 and organic dyes.31,32 Moreover, the spinel crystal structure of ferrites offers the availability of extra catalytic sites by virtue of the crystal lattice to enhance the photodegradation efficiency of pollutants.33,34 In particular, spinel ZnFe2O4, a magnetic material with a narrow bandgap of 1.9 eV, has been used for a wide variety of applications including water splitting, dye sensitized solar cells and photodegradation.34–36 However, the low VB potential and poor photoelectric conversion efficiency makes ZnFe2O4 an inferior photocatalyst toward pollutant degradation.37,38 Previous studies have shown that the coupling of ZnFe2O4 with optoelectronic materials such as TiO2, ZnO and graphene can form a new type of nanocomposite with good photocatalytic activity and separation properties.39–46 It is noteworthy that ZnFe2O4 is a p-type visible-light-driven semiconductor while anatase TiO2 is a well-known n-type semiconductor with indirect bandgap and long electron–hole life time.47,48 This gives a great impetus to fabricate a novel visible-light-responsive heterostructure photocatalyst by combining ZnFe2O4 with anatase TiO2. However, the fabrication of ZnFe2O4–TiO2 nanocomposites by coupling trace amounts of ZnFe2O4 with pure anatase TiO2 has received less attention. In addition, the effect of the amount of ZnFe2O4 added on the photocatalytic activity of heterostructure photocatalysts remains unclear.
Herein, a novel ZnFe2O4–TiO2 nanocomposite has been fabricated for the enhanced visible-light-responsive photodegradation of organic dyes including neutral rhodamine B (RhB), cationic methyl red (MR) and anionic methyl orange (MO) in the presence of 465 nm visible light. A non-aqueous hydrothermal method was used to fabricate nanoscale ZnFe2O4 particles at low mass loadings of 0.2–2 wt% and then coupled with pure anatase ST01 TiO2 for optimization. Morphology and surface characterization confirms that ZnFe2O4 is well distributed with TiO2. ZnFe2O4 in the nanocomposites can be excited by visible light and then anatase TiO2 serves as a charge carrier to participate in the surface reaction, resulting in the enhancement of photocatalytic activity of ZnFe2O4–TiO2 heterostructures toward dye decomposition. To the best of our knowledge, this is the first report of the optimization of the mass ratio of zinc ferrite to enhance the photocatalytic degradation efficiency of organic dyes by TiO2 based nanomaterials under visible light irradiation. In addition, the excellent reusability of nanocomposites indicates that ZnFe2O4–TiO2 has the potential to utilize visible light in a wide variety of environmental and energy applications.
2. Materials and methods
2.1. Synthesis of zinc ferrite (ZnFe2O4)
ZnFe2O4 nanoparticles were first synthesized using a non-aqueous hydrothermal method. Briefly, 10 mmol of NaOH was dissolved in 2 mL of bidistilled deionized water (Millipore Co, 18.3 MΩ cm) in an autoclave tube with a capacity of 40 mL. After the addition of 10 mL of 1-pentanol (C5H12O) under vigorous stirring, 0.5 mmol of oleic acid (C18H34O2) and 0.5 mmol of oleylamine (C18H37N), which serve as capping agents, were introduced into the solutions. A total of 2 mmol of iron nitrate (Fe(NO3)3·9H2O) and 1 mmol of zinc nitrate (Zn(NO3)2·6H2O) was dissolved in 14 mL of bidistilled deionized water which was then poured into the above solutions. After mixing well for 1 h, the mixture was heated to 180 °C for 16 h, cooled down to room temperature, and then the ZnFe2O4 nanoparticles were magnetically harvested from the liquid phase using a permanent magnet. The nanoparticles were washed with an n-hexane/ethanol (3/1, v/v) mixture three times to remove excess capping agent on the surface. Finally, ZnFe2O4 nanoparticles were re-dispersed in n-hexane, transferred to a watch glass, and dried in a vacuum oven at 60 °C for 6 h.2.2. Synthesis of ZnFe2O4–TiO2 nanocomposites
The ZnFe2O4–TiO2 nanocomposite photocatalysts were synthesized by mixing 80 mg of ST01 TiO2 (Ishihara Sangyo Ltd.) with various mass loadings of ZnFe2O4 at 0.2–2 wt% in 20 mL of octanol (C8H18O) and then sonicated for 1 h. The mixture was transferred into a 40 mL autoclave tube and heated to 240 °C for 2 h. The mixture was again cooled down to room temperature and then centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min to harvest the nanocomposites. Similar to the preparation procedure of ZnFe2O4, the ZnFe2O4–TiO2 precipitates were washed with a mixture of n-hexane/ethanol three times, and dried in a vacuum oven at 60 °C for 6 h.
000 rpm for 15 min to harvest the nanocomposites. Similar to the preparation procedure of ZnFe2O4, the ZnFe2O4–TiO2 precipitates were washed with a mixture of n-hexane/ethanol three times, and dried in a vacuum oven at 60 °C for 6 h.
2.3. Characterization
The surface morphology of the as-synthesized ZnFe2O4–TiO2 was examined by using scanning electronic microscopy (SEM) (Hitachi S-4800) with an acceleration electron voltage of 15 kV. Transmission electron microscopy (TEM) (Hitachi H-7100 TEM) and high resolution TEM (HRTEM) (FEI Tecnai G2) at 200 and 300 kV, respectively, were used to determine the dimensions and morphology of ZnFe2O4–TiO2. The distribution of elements was determined by an electron probe X-ray microanalyzer (JEOL JXA-8200) with an accelerating voltage and beam current 20 kV and 20 nA. The Brunauer–Emmett–Teller (BET) specific surface area and pore size distribution were determined by nitrogen adsorption and desorption at 77 K using a surface area and porosimetry system (ASAP 2020, Micromeritics). The crystallinity of the ZnFe2O4–TiO2 nanocomposites were identified by a Bruker NEW D8 ADVANCE X-ray diffractometer (XRD) with a Lynxeye high-speed strip detector and Ni-filtered Cu Kα radiation (λ = 1.5405 Å) operated at a generator voltage and an emission current of 40 kV and 40 mA, respectively. XRD patterns were recorded over a 2θ range of 20–80° at a sampling step width 0.05° (step time = 0.5 s). X-ray photoelectron spectroscopy (XPS) measurements were performed using an ESCA PHI 1600 photoelectron spectrometer (Physical Electronics, Eden Prairie, MN) using an Al Kα X-ray source at 1486.6 ± 0.2 eV. The optical spectra were examined using a Hitachi U-4100 UV-vis spectrophotometer in the wavelength range of 200–800 nm equipped with an integrating sphere accessory for diffuse reflectance spectra. Photoluminescence (PL) spectra were recorded on a Hitachi F-7000 fluorescence spectrometer with a 150 W xenon lamp as the excitation source.The photocurrent was determined by using a three-electrode system connected to an electrochemical work station (Autolab PGSTAT 302N) in the presence of 0.2 M Na2S2O4 as the electrolyte. A ZnFe2O4–TiO2/FTO electrode was used as the working electrode, and an Ag/AgCl electrode and platinum wire were used as the reference and counter electrodes, respectively. The transient photocurrents of pure ZnFe2O4, commercial TiO2 and ZnFe2O4–TiO2 nanocomposites were measured at an applied potential of 1 V under visible light irradiation. The surface charge (zeta potential) of ZnFe2O4–TiO2 nanocomposites was determined using a ZetaSizer Nano ZS (Malvern Instruments Inc., UK).
2.4. Photodegradation of organic dyes by ZnFe2O4–TiO2
The visible-light-responsive activity of ZnFe2O4–TiO2 was examined by using RhB, MR and MO as the target compounds in a hollow cylindrical photoreactor surrounded by eight 8 W visible lamps (λ = 465 ± 40 nm). In each experiment, the as-synthesized photocatalysts as well as commercial TiO2 nanoparticles were added into 20 mL of 10 mg L−1 dye solutions to get a final concentration of 1 g L−1. Prior to the photocatalytic reaction, the mixtures were mixed well under vigorous stirring conditions in the dark for 60 min and at 25 °C to avoid the interference of adsorption during the photocatalytic reactions. After the adsorption equilibrium, the visible light was switched on for photodegradation and the change in dye concentration in the supernatants was optically monitored using a UV-Vis spectrophotometer after the removal of photocatalysts by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min.
000 rpm for 5 min.
3. Results and discussion
3.1. Characterization of ZnFe2O4–TiO2 nanocomposites
In this study, various amounts of ZnFe2O4 were added as dopants to improve the visible-light-responsive activity of TiO2. Fig. 1 shows the TEM images of ZnFe2O4–TiO2 nanocomposites with various ZnFe2O4 amounts of 0–2 wt%. The TEM images show that the ZnFe2O4–TiO2 nanocomposites are regular shaped crystallites (Fig. 1a–d). However, the ZnFe2O4–TiO2 nanoparticles agglomerate to some extent, presumably attributable to the magnetic properties of the ZnFe2O4 particles. The SEM images displayed in Fig. S1 (ESI†) clearly show a slight increase in the particle size of ZnFe2O4–TiO2 nanocomposites when the loading amount of ZnFe2O4 increases from 0.5 to 2 wt%. The histogram shown in Fig. S2 (ESI†) indicates that the particle sizes of the ZnFe2O4–TiO2 nanocomposites at 0.2–2 wt% ZnFe2O4 are in the range of 7–11 nm. However, the mean particle size increases slightly from 7.5 nm at 0.2 wt% to 8.7 nm at 2 wt%, which is in good agreement with the results obtained from the SEM images.The HRTEM image of the 1 wt% ZnFe2O4–TiO2 nanocomposite shows two distinct lattices with d-spacings of 0.25 and 0.35 nm, which correspond to the (311) reflection of ZnFe2O4 and (101) reflection of anatase TiO2, respectively (Fig. 1e). It is clear that both ZnFe2O4 and TiO2 are nanosized particles with an average particle size of 8 nm. The EDS spectrum indicates the existence of Ti, Zn and Fe elements and that the weight ratio is 0.26 wt% for Zn and 0.48 wt% for Fe (Fig. 1f). The Fe/Zn molar ratio of 2.1 clearly indicates the formation of ZnFe2O4. In addition, the ZnFe2O4 nanoparticles are tightly linked with TiO2 nanoparticles. Since both TiO2 and ZnFe2O4 have similar particle sizes and are initially in contact with each other, the ZnFe2O4–TiO2 nanocomposites may be favorable for the charge transfer between ZnFe2O4 and TiO2, and result in the promotion of electron–hole pair separation as well as the enhancement of photocatalytic performance.
The elemental species in the ZnFe2O4–TiO2 nanomaterials were further identified using EPMA. Fig. S3 (ESI†) shows the elemental images of Ti, Zn, Fe and O in the 1 wt% ZnFe2O4–TiO2 nanocomposites. The uniform distribution of Ti and O atoms indicates that TiO2 nanoparticles are well distributed. In addition, the distribution of Zn and Fe are quite homogeneous with low intensity when compared with those of Ti and O atoms, presumably attributed to the low loading amount of ZnFe2O4.
The N2 adsorption–desorption isotherms of ZnFe2O4 and ZnFe2O4–TiO2 nanocomposites with 0.2–2 wt% ZnFe2O4 exhibit similar type IV isotherms with hysteresis loops in the relative pressure P/P0 within the range of 0.7–0.95 (Fig. S4a, ESI†), indicating the presence of mesopores in the nanocomposites. The specific surface area of ZnFe2O4–TiO2 increased from 123 m2 g−1 at 0.2 wt% ZnFe2O4 to 178 m2 g−1 at 1.5 wt% ZnFe2O4, and then slightly decreased to 164 m2 g−1 at 2 wt% ZnFe2O4. It is noteworthy that the specific surface area of pure ZnFe2O4 is 113 m2 g−1, showing that the combination of TiO2 with ZnFe2O4 can increase the specific surface area. In addition, the pore size distribution of pure ZnFe2O4 is in the range from 2–18 nm with an average pore diameter of 12 nm (Fig. S4b, ESI†). The ZnFe2O4–TiO2 nanocomposites also show a major pore size distribution in the range of 4–15 nm. However, another small pore size distribution ranging between < 1.7–4 nm was also observed for 0.5–2 wt% ZnFe2O4, clearly showing that the increase in the specific surface area of ZnFe2O4–TiO2 nanocomposites is mainly attributed to the increase in mesopores.
The crystallinity of pure ZnFe2O4, TiO2 and various ZnFe2O4 loadings of ZnFe2O4–TiO2 nanocomposites were further identified. As shown in Fig. 2, several diffraction peaks are centered at 2θ values of 29.89°, 35.24°, 42.97°, 53.16°, 56.79° and 62.28°, which are characteristic peaks of (220), (311), (400), (422), (511) and (440) orientations of ZnFe2O4, respectively (JCPDS 74-2397). The average grain size of ZnFe2O4, calculated from the Scherrer equation, is around 8 nm. In addition, the XRD patterns of ZnFe2O4–TiO2 nanocomposites show the major peaks of anatase phase TiO2 at 2θ values of 25.28°, 48.37°, 53.88°, 55.29°, 62.73°, 68.99°, 70.17°, and 75.37° (JCPDS 21-1272) and a small peak of ZnFe2O4 at 2θ = 35.24°, indicating that the composites can retain the crystallinity of both nanoparticles. It is noteworthy that only few peaks of ZnFe2O4 are identifiable in the XRD patterns, presumably attributable to the low loading amount of ZnFe2O4 onto TiO2. In addition, no rutile peak of TiO2 is observed for all of the as-synthesized ZnFe2O4–TiO2 nanocomposites, showing that the crystallinity of anatase phase of ST01 TiO2 can be retained during the preparation procedure.
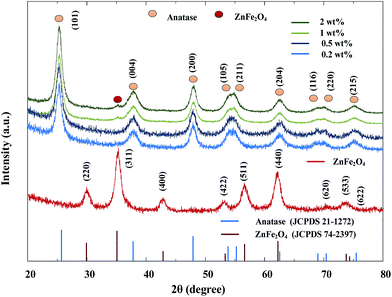 | ||
| Fig. 2 The XRD patterns of pure ZnFe2O4, TiO2 and ZnFe2O4–TiO2 nanocomposites with various mass loadings of ZnFe2O4 ranging from 0.5 to 2 wt%. | ||
The XPS spectra were further characterized to understand the chemical states of elements in the ZnFe2O4–TiO2 nanocomposites. The survey scan illustrated in Fig. 3a shows the coexistence of Zn 2p, Fe 2p, Ti 2p and O 1s elements in the as-synthesized ZnFe2O4–TiO2 nanocomposites. In addition, the existence of C 1s at 285 eV is ascribed to adventitious carbons from ambient environments. The high resolution XPS spectrum of Ti 2p shows two peaks located at 463.7 eV and 457.7 eV, which can be assigned to the Ti4+ peak of anatase TiO2 (Fig. 3b).39 The peaks at 1020.6 and 1044.1 eV shown in Fig. 3c are attributed to Zn2+ at tetrahedral sites. Peaks of Fe 2p at 710.2 and 724.5 eV are the typical 2p3/2 and 2p1/2 of iron oxide, respectively. In addition, peaks at 719.2 and 732.8 eV are the satellite peaks of iron oxides, which confirm the existence of Fe3+.49 Deconvolution of the O 1s region of the high-resolution XPS spectrum shows the typical peaks of metal–oxygen bonds of TiO2 and ZnFe2O4 (Fig. 3d),49 clearly indicating the coexistence and interaction of ZnFe2O4 and TiO2 in the composites.
 | ||
| Fig. 3 The (a) full survey XPS spectra and (b) Ti 2p, (c) Fe 2p (d) Zn 2p and (e) O 1s peaks of the 1 wt% ZnFe2O4–TiO2 nanocomposite. | ||
The UV-visible and PL spectra of the ZnFe2O4–TiO2 nanocomposites were determined to understand the optical properties of the nanocomposites. Fig. 4a presents the absorbance of ZnFe2O4–TiO2 nanocomposites with various loading amounts of ZnFe2O4 ranging from 0.2 to 2 wt%. It is clear that pure ZnFe2O4 shows a high absorption intensity in the visible light region. The absorption edge of pure ST01 TiO2 starts to increase at 380 nm, which is coincident with the reported result for anatase TiO2.50 Addition of ZnFe2O4 red-shifts the absorbance edge of the ZnFe2O4–TiO2 nanocomposites to the long wavelength region when the added amount of ZnFe2O4 increases from 0.2 to 2 wt%. Since TiO2 is an indirect band gap semiconductor, Tauc plots can be used to calculate the bandgaps of the ZnFe2O4–TiO2 nanocomposites.51 As shown in Fig. S4 (ESI†), the bandgap of pure ST01 TiO2 is 3.18 eV, which then decreases to 2.88–2.56 eV when the amount of ZnFe2O4 added increases from 0.2 to 2 wt%. It is noteworthy that the narrow band gap of ZnFe2O4 at 1.9 eV may induce lattice defects in anatase TiO2 under UV-visible light irradiation, which can serve as a center of exciton binding to enhance the photoactivity of ZnFe2O4–TiO2 nanocomposites.52
Fig. 4b shows the PL spectra of pure TiO2 and ZnFe2O4–TiO2 nanocomposites at various loading amounts of ZnFe2O4 in the wavelength range of 300–500 nm after excitation at 285 nm. The PL spectra of all the ZnFe2O4–TiO2 nanocomposites show a major peak at 315 nm, which is mainly attributed to the recombination of holes and electrons in the valence and conduction bands, respectively. The emission intensity at 315 nm decreases with an increase in ZnFe2O4 loading from 0 to 1 wt%, and then increases again slightly at 2 wt% ZnFe2O4. It is noteworthy that the PL spectra can be used to understand the behavior of electron–hole pairs on trapping, migration, and transfer properties.53,54 Since the emission signal at 315 nm originates from the recombination of excited electrons and holes, the decrease in fluorescence intensity is highly relevant to the low recombination rate of holes and electrons. This indicates that the 1 wt% ZnFe2O4–TiO2 nanocomposite may exhibit superior photocatalytic activity toward pollutant degradation than that of the other nanocomposites. In addition, the separation efficiency of photo-induced electron–hole pairs can be compared by the transient photocurrent response in several 100 s on-off intermittent irradiation cycles. As shown in Fig. 4c, pure ZnFe2O4 and commercial TiO2 nanoparticles including P25 and ST01 TiO2 show a low transient photocurrent and the photocurrent produced follows the order ZnFe2O4 < ST01 TiO2 < P25 TiO2, which is closely related to their bandgaps. On the contrary, the 1 wt% ZnFe2O4–TiO2 nanocomposite shows a better photoresponse than the other photocatalysts and the photocurrent density can be maintained at 5.9 μA cm−2 during the 4 cycles. This result clearly indicates that the 1 wt% ZnFe2O4–TiO2 nanocomposite can exhibit a long photoinduced carrier lifetime, which subsequently results in a high photocatalytic activity toward organic dye degradation.
3.2. The visible-light-responsive activity of ZnFe2O4–TiO2 toward organic dye degradation
The visible-light-sensitive activity of ZnFe2O4–TiO2 nanocomposites was first evaluated using RhB as the target compound under 465 nm visible light irradiation. As shown in Fig. 5a, only 1.4% of RhB is photodegraded after 60 min of visible light irradiation in the absence of a ZnFe2O4–TiO2 photocatalyst (direct photolysis). Pure anatase ST01 TiO2 shows little photocatalytic activity toward RhB degradation under 465 nm visible light irradiation, presumably attributable to its wide bandgap of 3.18 eV. The addition of ZnFe2O4 has a significant impact on the efficiency and rate of RhB photodegradation and 92.6% of the initial RhB is photodegraded by 0.2 wt% ZnFe2O4–TiO2. The removal efficiency of RhB increases upon increasing the loading amounts of ZnFe2O4 from 0.5 to 1 wt% and then slightly decreases when further increasing the ZnFe2O4 amount to 2 wt%. The addition of 1 wt% ZnFe2O4–TiO2 shows superior photocatalytic activity toward RhB degradation when compared to other added amounts of ZnFe2O4, which is in good agreement with the optical data obtained from the PL spectra.It is noteworthy that the equilibrium of 60 min between organic dye and various loading amounts of ZnFe2O4–TiO2 causes a 30–48% decrease in RhB concentration prior to photodegradation, showing that adsorption is a possible mechanism for RhB removal. To further understand the adsorption effect on the removal of RhB by TiO2-based nanomaterials, adsorption of RhB by commercial TiO2 and 1 wt% ZnFe2O4–TiO2 was performed in the dark. As shown in Fig. S5 (ESI†), less than 5% of RhB concentration were adsorbed in solutions containing commercial TiO2 products including Degussa P25 and ST01 TiO2 after 120 min of adsorption in the dark. In the presence of 1 wt% ZnFe2O4–TiO2, however, the concentration of RhB decreases with time during the first 60 min and a total of 48% of RhB is adsorbed by 1 wt% ZnFe2O4–TiO2 after 120 min of incubation. The adsorption of RhB is mainly attributed to the fact that ZnFe2O4–TiO2 is fabricated using a non-aqueous hydrothermal method and would contain some organic residues onto the surface after phase transfer. In addition, the pHIEP of 1 wt% ZnFe2O4–TiO2 is 6.7 (Fig. S6, ESI†), which means that the ZnFe2O4–TiO2 is negatively charged at pH 7. Therefore, the cationic RhB would adsorb onto the surface of ZnFe2O4–TiO2. These results clearly indicate that the adsorption can be equilibrated within 60 min prior to photodegradation and has little influence on the photodegradation efficiency and rate of RhB.
The photocatalytic degradation of organics by TiO2-based nanomaterials usually obeys pseudo-first order kinetics.55 In this study, the decrease in RhB concentration follows adsorption first and then undergoes photodegradation. After subtracting the adsorption part, the photocatalytic degradation of RhB follows pseudo-first order kinetics and the rate constants (k) for RhB photodegradation by 0.2, 0.5, 1, and 2 wt% ZnFe2O4–TiO2 are 0.038 (r2 = 0.998), 0.069 (r2 = 0.986), 0.091 (r2 = 0.993) and 0.078 (r2 = 0.996) min−1, respectively (Fig. 5b). The UV-visible spectra of aqueous RhB solutions in the presence of 1 wt% ZnFe2O4–TiO2 under 465 nm visible light irradiation show that the intensity of absorption peak at 554 nm decreases rapidly with time, indicating the excellent visible-light-responsive activity of the ZnFe2O4–TiO2 nanocomposites (Fig. S6, ESI†). It is noteworthy that the maximum absorption peak blue-shifts from 554 to 498 nm after 60 min of visible light irradiation. A previous study has investigated the decomposition of RhB by N-doped TiO2 based nanoparticles and found that de-ethylated intermediates could cause the shift in absorption band during photodegradation,56 which is in good agreement with the results obtained in this study.
To further understand the photocatalytic activity of ZnFe2O4–TiO2 toward different types of organic dyes, photodegradation experiments were performed by selecting methyl red (MR) and methyl orange (MO) as neutral and anionic dyes, respectively. In addition, 1 wt% ZnFe2O4–TiO2 was selected as the photocatalyst for further experiments. Fig. 6 shows the photocatalytic degradation efficiency and rate of MR and MO by 1 wt% ZnFe2O4–TiO2 under visible light irradiation. The photodegradation efficiencies of MR by commercial ST-01 and Degussa P-25 TiO2 are 10% and 40%, respectively (Fig. 6a). In addition, pure ZnFe2O4 shows only a slight effect on photodegradation on the three organic dyes under the same experimental conditions (Fig. S9, ESI†). Addition of 1 wt% ZnFe2O4–TiO2 enhances the photocatalytic degradation efficiency of MR and nearly complete photodegradation is observed within 90 min of irradiation (Fig. 6a). However, only 82% of MO is photodegraded by ZnFe2O4–TiO2 after 90 min of irradiation (Fig. 6b). It is noteworthy that 20% of MR and 22% of MO are adsorbed by ZnFe2O4–TiO2 in the dark. The lesser amounts of MR and MO adsorbed compared with that of RhB is mainly due to the negatively charged surface of ZnFe2O4–TiO2. In addition, the molecular structure of organic dyes shows a great impact on the photocatalytic activity of ZnFe2O4–TiO2 and the kobs for dye photodegradation is 0.0445 min−1 for MR and 0.0136 min−1 for MO. This result clearly indicates that the photodegradation rate of organic dyes by ZnFe2O4–TiO2 follows the order RhB > MR > MO (Fig. 7c). It is noteworthy that the photocatalytic degradation of organic chemicals is a surface-mediated reaction,7 and the adsorption of organic dyes onto the surface of ZnFe2O4–TiO2 is the first step for photodegradation. Cationic RhB can easily be adsorbed onto the negatively charged ZnFe2O4–TiO2 and shows excellent photocatalytic efficiency and rate compared to neutral MR and anionic MO. Therefore, RhB is selected for further experiments because of the excellent photocatalytic degradation efficiency and rate.
 | ||
| Fig. 6 The photodegradation of (a) neutral methyl red (MR) and (b) anionic methyl orange (MO) in the presence of 1 wt% ZnFe2O4–TiO2 under visible light irradiation. | ||
3.3. Effect of RhB concentration on photodegradation
The photodegradation of various initial concentrations of RhB was further examined. Fig. 7 shows the photodegradation of various initial concentrations of RhB by 1 wt% ZnFe2O4–TiO2 under 465 nm visible light irradiation. It is evident that the photodegradation efficiency of RhB decreases with the increase in initial RhB concentrations ranging from 5 to 40 mg L−1. As shown in Fig. 7a, a nearly complete photodegradation of 5 mg L−1 RhB by 1 wt% ZnFe2O4–TiO2 is observed after 60 min of irradiation. However, only 79% of the original RhB is photodecomposed when the RhB concentration is increased to 40 mg L−1. The photodegradation of RhB at various initial concentrations also follows pseudo-first order kinetics and the kobs for RhB photodegradation decreases from 0.1687 to 0.0229 min−1 when the initial concentration increases from 5 to 40 mg L−1 (Fig. 7b). The decrease in kobs for RhB photodegradation at high initial concentrations is possibly attributable to the inhibition effect of adsorbed RhB molecules on light penetration. It is noteworthy that RhB is a red-colored dye and that an increase in RhB concentration would absorb more light. Therefore, an increase in initial RhB concentration decreases the penetration of photons to the photocatalyst surface, resulting in a decrease of the photocatalytic degradation efficiency and rate of RhB.57The limited number of reactive sites on the ZnFe2O4–TiO2 nanocomposites is another plausible reason for the decreased rate constants. Our previous studies have indicated that the photocatalytic degradation of organic pollutants by photocatalysts follows a surface-mediated process and Langmuir–Hinshelwood kinetics can be employed to describe the relationship:7
 | (1) |
3.4. Reusability of ZnFe2O4–TiO2
Recovery and reusability are also important parameters for evaluating the possible application of photocatalysts. In this study, the photocatalytic reusability of ZnFe2O4–TiO2 was further examined by repeated injection of 10 mg L−1 RhB under visible light irradiation. As shown in Fig. 8, RhB can be rapidly photodegraded by 1 wt% ZnFe2O4–TiO2 within 60 min. After the re-injection of the same concentration of RhB into the solutions, ZnFe2O4–TiO2 still exhibits excellent visible-light-responsive activity and can effectively photodegrade RhB to >99% for at least 5 cycles. The kobs for RhB photodegradation decreases from 0.0908 min−1 for the first cycle to 0.0589 min−1 at the 5th addition. The decrease in rate constant is mainly attributed to the accumulation of intermediates after the photocatalytic degradation of RhB, which is in good agreement with the UV-visible spectra shown in Fig. S7 (ESI†).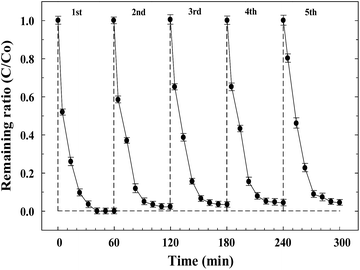 | ||
| Fig. 8 The photodegradation of rhodamine B (RhB) by the recycled 1 wt% ZnFe2O4–TiO2 under 465 nm visible light irradiation. | ||
3.5. Possible reaction mechanism
In this study, the ZnFe2O4–TiO2 nanocomposites show excellent photocatalytic activity toward organic dye degradation. It is known that TiO2 is a UV-sensitive photocatalyst and the addition of the narrow bandgap material ZnFe2O4 can enhance the visible-light-responsive properties of the nanocomposites. As shown in Scheme 1, electrons in the VB of ZnFe2O4 can be photoexcited to the CB after the irradiation of visible light, and result in the production of electron–hole pairs. Since the energy position of the CB of ZnFe2O4 at −1.54 eV is higher than that of anatase TiO2 (−0.30 eV),58 the excited electrons in ZnFe2O4 can be easily transferred across the interface of nanocomposites to the CB of anatase TiO2, and leaves holes in the VB of ZnFe2O4. Therefore, the coupling of ZnFe2O4 and TiO2 can effectively reduce the recombination rate of electrons and holes, which subsequently enhances the interfacial charge transfer efficiency. It is noteworthy that the photogenerated holes (h+) in the valence band of ZnFe2O4 cannot react with surface-absorbed H2O to generate highly reactive hydroxyl radicals (˙OH) because of the lower valence band position (0.38 eV) in comparison with that of water oxidation [E0(˙OH/OH−) = 2.38 V]. In contrast, electrons in the conduction band of TiO2 at pH 7 are at −0.29 V which can provide sufficient reducing power to generate superoxide anion (O2−˙) and peroxyl (HO2˙) radicals [E0(O2/O2−˙) = −0.33 V and E0(O2/HO2˙) = −0.05 V]. The oxygen-containing radicals (O2−˙ and HO2˙) produced can further react with electrons and protons to produce hydroxyl radicals (˙OH), resulting in an enhanced photodegradation efficiency and rate for RhB and other organic dyes under visible light irradiation. It is noteworthy that Z-scheme electron transfer can occur when the two narrow bandgap photocatalysts are excited by visible light. During Z-scheme electron transfer, the photogenerated electrons in the low CB of photocatalyst I are transferred to the high VB of photocatalyst II through an electron mediator.59 In this study, only ZnFe2O4 can be excited by 465 nm visible light, and then the photogenerated electrons in the CB of ZnFe2O4 transfer to the CB of TiO2. Since an electron mediator is lacking which can transfer electrons from the CB of TiO2 to the VB of ZnFe2O4, the electron transfer mechanism in this study bears more resemblance to that of a heterostructured photocatalytic system.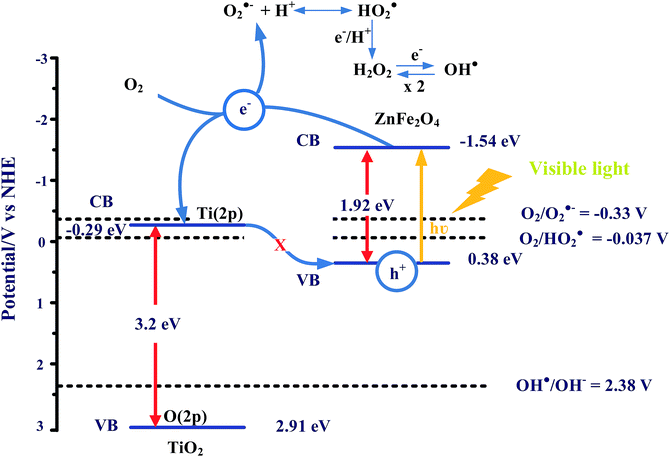 | ||
| Scheme 1 The possible photocatalytic mechanism for organic dye degradation by ZnFe2O4–TiO2 under visible light irradiation. | ||
To demonstrate the generation of hydroxyl radicals (˙OH) by visible light irradiation, terephthalic acid (TPA), a fluorescent probe which can readily react with hydroxyl radicals to produce a highly fluorescent product 2-hydroxy terephthalic acid (TAOH) at 425 nm, was used in the ZnFe2O4–TiO2 system under 465 nm visible light irradiation.60 Fig. S8 (ESI†) shows the change in the PL spectra of TAOH after the visible light irradiation of 1 wt% ZnFe2O4–TiO2 in the presence of 5 × 10−4 M TPA solutions. It is clear that the PL intensity at 425 nm increases with time when ZnFe2O4–TiO2 nanocomposites are irradiated by visible light. However, no PL peak at 425 nm is observed for ZnFe2O4–TiO2 in the absence of visible light, clearly indicating the production of ˙OH by the ZnFe2O4–TiO2 nanocomposites under visible light irradiation.
4. Conclusions
In this study, we have successfully fabricated a novel ZnFe2O4–TiO2 nanocomposite with various mass loadings of 0.2–2 wt% ZnFe2O4 for the enhanced photocatalytic degradation of organic dyes under 465 nm visible light irradiation. The TEM images show that the average particle sizes of ZnFe2O4 and anatase TiO2 nanoparticles are 8–9 nm. The optical properties clearly indicate possible electron transfer at the interface of ZnFe2O4 and anatase TiO2 nanoparticles, resulting in the acceleration of photodegradation efficiency and rate of organic dyes under visible light irradiation. The photodegradation rate of organic dyes by 1 wt% ZnFe2O4–TiO2 follows the order RhB > MR > MO. In addition, the 1 wt% ZnFe2O4–TiO2 can retain its photocatalytic efficiency for at least 5 reaction cycles with a stable degradation efficiency of >99% under visible light irradiation. Our results clearly demonstrate that the combination of a low ZnFe2O4 mass with anatase TiO2 is a reliable green technology approach with great potential for applications in utilizing visible light to decompose recalcitrant and emerging pollutants in the treatment of water and wastewater.Acknowledgements
The authors thank the Ministry of Science and Technology (MOST), Taiwan for financial support under grant No. MOST 104-2221-E-009-020-MY3.Notes and references
- W. Wu, C. Z. Jiang and V. A. L. Roy, Nanoscale, 2015, 7, 38–58 CAS.
- M. A. Lazar, S. Varghese and S. S. Nair, Catalysts, 2012, 2, 572–601 CrossRef CAS.
- J. Y. Gan, X. H. Lu and Y. X. Tong, Nanoscale, 2014, 6, 7142–7164 RSC.
- S. A. Ansari and M. H. Cho, Sci. Rep., 2016, 6, 25405 CrossRef CAS PubMed.
- R. Sedghi and F. Heidari, RSC Adv., 2016, 6, 49459–49468 RSC.
- Y. Luo, Z. Lu, Y. Jiang, D. Wang, L. Yang, P. Huo, Z. Da, X. Bai, X. Xie and P. Yang, Chem. Eng. J., 2014, 240, 244–252 CrossRef CAS.
- C. Han, N. Zhang and Y.-J. Xu, Nano Today, 2016, 11, 351–372 CrossRef CAS.
- S. Liu, C. Han, Z. R. Tan and Y. J. Xu, Mater. Horiz., 2016, 3, 270–282 RSC.
- M. Q. Yang and Y. J. Xu, Nanoscale Horizons, 2016, 1, 185–200 RSC.
- R. A. Doong, S. M. Chang and C. W. Tsai, Appl. Catal., B, 2013, 129, 48–55 CrossRef CAS.
- R. A. Doong and C. W. Tsai, J. Taiwan Inst. Chem. Eng., 2015, 57, 69–76 CrossRef CAS.
- Y. B. Liu, Q. F. Yao, X. J. Wu, T. K. Chen, Y. Ma, C. N. Ong and J. P. Xie, Nanoscale, 2016, 8, 10145–10151 RSC.
- S. Semlali, T. Pigot, D. Flahaut, J. Allouche, S. Lacombe and L. Nicole, Appl. Catal., B, 2014, 150, 656–662 CrossRef.
- P. Yu, S. S. Wang, H. H. Li and W. X. Li, Adv. Environ. Technol., 2013, 726–731, 673–676 Search PubMed.
- B. Sambandam, A. Surenjan, L. Philip and T. Pradeep, ACS Sustainable Chem. Eng., 2015, 3, 1321–1329 CrossRef CAS.
- P. Xu, T. Xu, J. Lu, S. M. Gao, N. S. Hosmane, B. B. Huang, Y. Dai and Y. B. Wang, Energy Environ. Sci., 2010, 3, 1128–1134 CAS.
- E. M. Samsudin, S. B. Abd Hamid, J. C. Juan, W. J. Basirun and G. Centi, Chem. Eng. J., 2015, 280, 330–343 CrossRef CAS.
- V. Stengl, S. Bakardjieva, N. Murafa, V. Houskova and K. Lang, Microporous Mesoporous Mater., 2008, 110, 370–378 CrossRef CAS.
- M. T. Uddin, Y. Nicolas, C. Olivier, T. Toupance, M. M. Mueller, H. J. Kleebe, K. Rachut, J. Ziegler, A. Klein and W. Jaegermann, J. Phys. Chem. C, 2013, 117, 22098–22110 CAS.
- J. Shen, Y. Meng and G. Xin, Rare Met., 2011, 30, 280–283 CrossRef CAS.
- Y. Xin, M. Gao, Y. Wang and D. Ma, Chem. Eng. J., 2014, 242, 162–169 CrossRef CAS.
- Y. Wang, Y. Z. Zheng, S. Q. Lu, X. Tao, Y. K. Che and J. F. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 6093–6101 CAS.
- D. L. Liao, C. A. Badour and B. Q. Liao, J. Photochem. Photobiol., A, 2008, 194, 11–19 CrossRef CAS.
- M. Khatamian, M. S. Oskoui, M. Haghighi and M. Darbandi, Int. J. Energy Res., 2014, 38, 1712–1726 CrossRef CAS.
- B. Wang, H. Zhang, X. Y. Lu, J. Xuan and M. K. H. Leung, Chem. Eng. J., 2014, 253, 174–182 CrossRef CAS.
- P. Khemthong, P. Photai and N. Grisdanurak, Int. J. Hydrogen Energy, 2013, 38, 15992–16001 CrossRef CAS.
- L. F. Chiang and R. A. Doong, J. Hazard. Mater., 2014, 277, 84–92 CrossRef CAS PubMed.
- C. Santhosh, P. Kollu, S. Felix, V. Velmurugan, S. K. Jeong and A. N. Grace, RSC Adv., 2015, 5, 28965–28972 RSC.
- I. L. Liakos, M. H. Abdellatif, C. Innocenti, A. Scarpellini, R. Carzino, V. Brunetti, S. Marras, R. Brescia, F. Drago and P. P. Pompa, Molecules, 2016, 21, 520–534 CrossRef PubMed.
- M. Kooti, P. Kharazi and H. Motamedi, J. Mater. Sci. Technol., 2014, 30, 656–660 CAS.
- H. Y. Zhu, R. Jiang, Y. Q. Fu, R. R. Li, J. Yao and S. T. Jiang, Appl. Surf. Sci., 2016, 369, 1–10 CrossRef CAS.
- A. Tadjarodi, M. Imani and M. Salehi, RSC Adv., 2015, 5, 56145–56156 RSC.
- R. Dom, R. Subasri, K. Radha and P. H. Borse, Solid State Commun., 2011, 151, 470–473 CrossRef CAS.
- J. Q. Pan, X. Y. Li, Q. D. Zhao and D. K. Zhang, RSC Adv., 2015, 5, 51308–51317 RSC.
- X. Y. Liu, H. W. Zheng, Y. Li and W. F. Zhang, J. Mater. Chem. C, 2013, 1, 329–337 RSC.
- Y. J. Yao, Y. M. Cai, F. Lu, J. C. Qin, F. Y. Wei, C. Xu and S. B. Wang, Ind. Eng. Chem. Res., 2014, 53, 17294–17302 CrossRef CAS.
- W. Q. Zhang, M. Wang, W. J. Zhao and B. Q. Wang, Dalton Trans., 2013, 42, 15464–15474 RSC.
- A. R. Upreti, Y. Li, N. Khadgi, S. Naraginti and C. Zhang, RSC Adv., 2016, 6, 32761–32769 RSC.
- X. Zhu, F. Zhang, M. Wang, J. Ding, S. Sun, J. Bao and C. Gao, Appl. Surf. Sci., 2014, 319, 83–89 CrossRef CAS.
- M. Y. Wang, L. Sun, J. H. Cai, P. Huang, Y. F. Su and C. J. Lin, J. Mater. Chem. A, 2013, 1, 12082–12087 CAS.
- Q. Q. Xu, J. T. Feng, L. C. Li, Q. S. Xiao and J. Wang, J. Alloys Compd., 2015, 641, 110–118 CrossRef CAS.
- Y. J. Yao, J. C. Qin, H. Chen, F. Y. Wei, X. T. Liu, J. L. Wang and S. B. Wang, J. Hard Mater., 2015, 291, 28–37 CrossRef CAS PubMed.
- G. X. Song, F. Xin and X. H. Yin, J. Colloid Interface Sci., 2015, 442, 60–66 CrossRef CAS PubMed.
- Z. H. Yuan and L. D. Zhang, J. Mater. Chem., 2001, 11, 1265–1268 RSC.
- Y. Bu, Z. Chen and W. Li, Dalton Trans., 2013, 42, 16272–16275 RSC.
- Y. Fu and X. Wang, Ind. Eng. Chem. Res., 2011, 50, 7210–7218 CrossRef CAS.
- M. K. Nowotny, P. Bogdanoff, T. Dittrich, S. Fiechter, A. Fujishima and H. Tributsch, Mater. Lett., 2010, 64, 928–930 CrossRef CAS.
- L. Kong, Z. Jiang, T. Xiao, L. Lu, M. O. Jones and P. P. Edwards, Chem. Commun., 2011, 47, 5512–5514 RSC.
- Y. Hou, X. Y. Li, Q. D. Zhao, X. Quan and G. H. Chen, Adv. Funct. Mater., 2010, 20, 2165–2174 CrossRef CAS.
- M. G. Mendez-Medrano, E. Kowalska, A. Lehoux, A. Herissan, B. Ohtani, D. Bahena, V. Briois, C. Colbeau-Justin, J. L. Rodriguez-Lopez and H. Remita, J. Phys. Chem. C, 2016, 120, 5143–5154 CAS.
- R. Pandiyan, N. Delegan, A. Dirany, P. Drogui and M. A. El Khakani, J. Phys. Chem. C, 2016, 120, 631–638 CAS.
- X. Li, Y. Hou, Q. Zhao and G. Chen, Langmuir, 2011, 27, 3113–3120 CrossRef CAS PubMed.
- S. A. Ansari, M. M. Khan, S. Kalathil, A. Nisar, J. Lee and M. H. Cho, Nanoscale, 2013, 5, 9238–9246 RSC.
- C. Jin, B. Liu, Z. Lei and J. Sun, Nanoscale Res. Lett., 2015, 10, 1–9 CrossRef CAS PubMed.
- L. F. Chiang and R. A. Doong, Sep. Purif. Technol., 2015, 156, 1003–1010 CrossRef CAS.
- D. Nassoko, Y. F. Li, H. Wang, J. L. Li, Y. Z. Li and Y. Yu, J. Alloys Compd., 2012, 540, 228–235 CrossRef CAS.
- S. Pitchaimuthu, S. Rajalakshmi, N. Kannan and P. Velusamy, Desalin. Water Treat., 2014, 52, 3392–3402 CrossRef CAS.
- S. Zhang, J. Li, M. Zeng, G. Zhao, J. Xu, W. Hu and X. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 12735–12743 CAS.
- P. Zhou, J. G. Yu and M. Jaroniec, Adv. Mater, 2014, 26, 4920–4935 CrossRef CAS PubMed.
- W. J. Ong, L. L. Tan, S. P. Chai and S. T. Yong, Chem. Commun., 2015, 51, 858–861 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images, particle size distribution, surface area, pore size distribution, EPMA images and Tauc plots of ZnFe2O4–TiO2; adsorption of rhodamine B by ZnFe2O4–TiO2; zeta potential of ZnFe2O4–TiO2; change in absorption spectra of rhodamine B after visible light irradiation; photodegradation of organic dye by pure ZnFe2O4, and PL spectra of 2-hydroxy terephthalic acid. See DOI: 10.1039/c6ra21002c |
| This journal is © The Royal Society of Chemistry 2016 |




