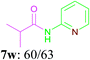Microwave-assisted heteropolyanion-based ionic liquid promoted sustainable protocol to N-heteroaryl amides via N-directing dual catalyzed oxidative amidation of aldehydes†
Renzhong Fu,
Yang Yang*,
Weihua Jin,
Hui Gu,
Xiaojun Zeng,
Wen Chai,
Yunsheng Ma ,
Quan Wang,
Jun Yi* and
Rongxin Yuan
,
Quan Wang,
Jun Yi* and
Rongxin Yuan
Jiangsu Laboratory of Advanced Functional Material, School of Chemistry and Materials Engineering, Changshu Institute of Technology, No. 99, 3rd South Ring Road, Changshu 215500, P. R. China. E-mail: yangyangirisjs@126.com
First published on 7th November 2016
Abstract
A sustainable procedure for the synthesis of N-heteroaryl amides directly from oxidative amidation of aldehydes catalyzed by heteropolyanion-based ionic liquids under microwave-promoted conditions has been reported. The transformation has proven to tolerate a wide range of aldehydes and amino heterocycles with different functional groups. Moderate to excellent yields, solvent-free media, operational simplicity and reusability of catalysts are the main highlights. Furthermore, the proposed N-directing dual-catalysis mechanistic pathway was briefly investigated in this report.
Introduction
Amide functionalities have attracted a great deal of attention in current chemical and industrial research not only owing to their remarkable biological and pharmacological activities,1,2 but also because they are versatile building blocks for the synthesis of various useful molecules.3–6 To our knowledge, more than 50% of known drugs contain amide units.7 Among amides, N-heteroaryl amides as an important class of pharmacophores are widely found in bioactive compounds.8–11 Recently, pyridyl benzamide derivative 1 was reported as inhibitors for the Trypanosoma brucei;8 tubulin polymerization inhibitor 2 showed tumor growth inhibition;9 thiazolyl amides 3 were selective inhibitors of the protein methyltransferase;10 compound 4 was allosteric glutaminase inhibitors based on N-heteroaryl amide scaffold (Fig. 1).Conventionally, N-heteroaryl amides are synthesized by acylation of amino heterocycles with activated carboxylic acid derivatives using suitable coupling reagents.12 Although novel coupling reagents13–18 and improved chemical processing techniques19 were subsequently developed, these methods have several innate drawbacks due to the weak nucleophilicity of amino heterocycles, such as harsh reaction conditions, toxic reagents, poor atom-efficiency, excess amount of by-products. To circumvent these limitations, several innovative strategies have been explored to improve N-heteroaryl amide derivatives synthesis, including amidation of heteroaryl halides,20–22 aminocarbonylation of aryl halides,23,24 trapping of ketenes with amino heterocycles,25 decomposition of N-heteroaryl-N′-benzoylthioureas26 and oxidative amidation methods.27–35 Among them, direct oxidative amidation of aldehydes with amino heterocycles is of great interest in synthetic organic chemistry due to atom economy and easily available substrates. To date, several metal catalyst systems have been reported to promote this oxidative amidation of aldehydes to achieved N-heteroaryl amides, such as CuI/air31–33 and Rh(III)/Ag2CO3.34 Just recently, Yadav et al. presented Cu(OTf)2/I2 as an effective catalyst for the oxidative amidation reaction from the view point of green chemistry introducing aqueous micellar reaction systems.35 However, these methodologies suffer from some common demerits that greatly restricted large scale industrial applications, such as requirement for toxic metal reagents and oxidants, narrow substrate scope, difficulties in separation and recycling of the catalysts. Therefore, there is an urgent need to develop more efficient and sustainable catalytic processes to a wide range of N-heteroaryl amides.
Over the last few decades, the development of more sustainable chemical and industrial processes was attractive due to global concern for environmental protection. So far, ionic liquids (ILs) as efficient and ecofriendly solvents and/or catalysts in organic reactions provide both economical and ecological benefits.36,37 Recently, a series of heteropolyanion-based ILs (HPAILs) have emerged as new species of hybrid materials, which were prepared by combining Keggin heteropolyanions with ‘task-specific’ IL (TSIL) cations containing special functional groups.38–40 In view of the advantages of heteropolyanions and TSIL cations, HPAILs generally have high melting points, thermal stability and chemical stability due to the large volume and high valence of heteropolyanions and hydrogen bonding networks existing in the compounds. In addition, protocols containing HPAILs are an attractive alternative for traditional acid-catalyzed41–46 or oxidative47–53 organic transformations because of their operational simplicity, no toxicity, easy isolation and reusability.
On the other hand, a large number of publications have clearly shown that many types of chemical transformations can be carried out successfully under microwave (MW) conditions during the last years.54 Most importantly, MW processing frequently leads to the significant rate enhancements, yield and selectivity improvements, very simplified ease of manipulation and work-up, less environmental polluting processes as well as solvent-free organic transformations matching with the goal of green chemistry.55,56 Our group has previously reported HPAILs catalyzed amidation via condensation using carboxylic acid derivatives and amines (Scheme 1(a)).57–59 Whereafter, HPAILs are introduced into catalyzed oxidative amidation by us, while only aliphatic amines were well tolerated in the reactions with limited substrate scope and practical application (Scheme 1(b)).60 As a part of our research to pursue novel, efficient and green methods for organic transformations,57–64 herein we wish to report the MW-assisted HPAILs catalyzed oxidative amidation of aldehydes with amino heterocycles to N-heteroaryl amides via N-directing dual catalysis process (Scheme 1(c)).
Results and discussion
Based on our previous investigations in HPAIL catalyzed organic reactions,57–60 N-substituted imidazole, pyridine and triethylamine based HPAILs were chosen as potential catalysts for this oxidative amidation (Fig. 2).Initially, our studies were commenced using commercially available benzaldehyde 5a and 2-aminopyridine 6a as the model substrates to optimize the reaction conditions (Table 1). Firstly, as a control experiment, tert-butyl hydroperoxide (TBHP) was used as the oxidant in the absence of any catalyst and additional solvent at room temperature. The complete lack of reactivity was observed even after stirring for 12 h (Table 1, entry 1), whereas addition of 3 mol% amount of [PyPS]3PW12O40 to the reaction mixture resulted in the desired N-pyridinamide 7a in 31% yield (Table 1, entry 2). The results revealed that oxidative amidation to N-heteroaryl amides could be promoted by HPAILs. In order to improve the yield, some adjustments to the reaction conditions were made. To our delight, it was shown that the rate and yield of the reaction both increased dramatically when the reaction mixture was conventionally heated at 50 °C (Table 1, entry 3, 54% yield) or 70 °C (Table 1, entry 4, 78% yield). In addition, more efficient results were observed when MW-assisted heating was introduced (Table 1, entries 3 and 4). Then reactions were performed using other common oxidants including H2O2, oxone, NaOCl, and m-CPBA, but in all cases only 0–5% amide product formation was observed (Table 1, entries 5–8). To our surprise, decreasing the catalyst loading to 2 mol% and 1 mol% led to the formation of the amide product in little higher yields, while much less amount (0.5 mol%) was not beneficial to the product (Table 1, entry 9–11). Afterwards the catalytic activities of other related catalysts prepared earlier were screened (Table 1, entry 12–16). PyPS species were found to be more efficient than MIMPS and TEAPS species, while the results demonstrated that PW12O40 heteropolyanions was more active than PMo12O40 heteropolyanions. Finally, the optimized condition for this oxidative amidation was shown using 1 mol% of [PyPS]3PW12O40 under MW and solvent-free conditions at 70 °C affording N-(pyridin-2-yl)benzamide in 86% yield (Table 1, entry 10).
| Entry | Catalyst [mol%] | Oxidant | Time (h)/min | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde 5a (2 mmol), 2-aminopyridine 6a (2.4 mmol), HPAIL catalyst and TBHP (1.5 equiv., 70% aqueous solution) under MW (700 W) and solvent-free conditions at 70 °C in a sealed glass pressure tube.b Isolated yields based on benzaldehyde 5a.c Reaction was conducted under room temperature.d Reaction was conducted under conventional heating at 50 °C.e Reaction was conducted under conventional heating at 70 °C. | ||||
| 1 | — | TBHP | (12)c | 0c |
| 2 | [PyPS]3PW12O40 [3] | TBHP | (12)c | 31c |
| 3 | [PyPS]3PW12O40 [3] | TBHP | (12)d, 50 | 54d, 61 |
| 4 | [PyPS]3PW12O40 [3] | TBHP | (12)e, 50 | 78e, 82 |
| 5 | [PyPS]3PW12O40 [3] | H2O2 | 50 | <5 |
| 6 | [PyPS]3PW12O40 [3] | Oxone | 50 | <5 |
| 7 | [PyPS]3PW12O40 [3] | m-CPBA | 50 | <5 |
| 8 | [PyPS]3PW12O40 [3] | NaOCl | 50 | <5 |
| 9 | [PyPS]3PW12O40 [2] | TBHP | 50 | 83 |
| 10 | [PyPS]3PW12O40 [1] | TBHP | 50 | 86 |
| 11 | [PyPS]3PW12O40 [0.5] | TBHP | 50 | 77 |
| 12 | [PyPS]3PMo12O40 [1] | TBHP | 50 | 78 |
| 13 | [MIMPS]3PW12O40 [1] | TBHP | 50 | 81 |
| 14 | [MIMPS]3PMo12O40 [1] | TBHP | 50 | 71 |
| 15 | [TEAPS]3PW12O40 [1] | TBHP | 50 | 40 |
| 16 | [TEAPS]3PMo12O40 [1] | TBHP | 50 | 36 |
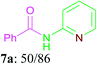
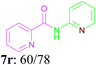
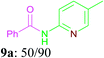
With the optimal conditions in hand, the scope and generality of this oxidative amidation were explored using various aldehydes and amino heterocycles. Initially, a set of aldehydes, including aromatic and aliphatic aldehydes, were employed in the reaction with moderate to good yields (Table 2). The results shown that the reaction could tolerate a variety of substituents (Me, OMe, F, Cl, Br, CO2Me, CF3, CN and NO2) on the aryl aldehyde moiety, which are very important functional units in pharmaceutical chemistry. In general, bearing electron-donating substitutions (Table 2, 7a–7e) on the aryl nucleus showed relatively higher reactivity than those of the electron-withdrawing (Table 2, 7f–7n) counterparts. While sterically hindered substituents such as MeO (Table 2, 7e) and Br (Table 2, 7j) at the ortho-position of aryl aldehydes gave the lower yield than para position and meta position substituted substrates. Following the aryl aldehydes, various heterocyclic aldehydes were well tolerated and furnished the corresponding products in moderate to good yields, including pyridinecarboxaldehyde, furfural and thiophenecarboxaldehyde (Table 2, 7o–7t). Subsequently, in the cases of aliphatic aldehydes, the lower yields (Table 2, 7u–7w) when compared to aromatic aldehydes may be due to the side reactions such as aldol condensation and oxidation to the corresponding carboxylic acids. Moreover, it was worth mentioning that N-(pyridin-2-yl)formamide was furnished via this oxidative amidation methodology in 76% yield, which normally showed poor yields in other reports.
With regard to the reactivities of amino heterocycles, 2-aminopyridines analogues containing H, CH3, Cl and NH2 substitutions exhibited moderate to good reactivity. It was shown that electron-donating substituents (Table 2, 8a–11a) on the pyridine ring were beneficial for the transformation, whereas electron-withdrawing substituents decreased the yield (Table 2, 12a–13a, NH2 group shows electron-withdraw in the acid catalyzed reaction conditions), while introducing steric hindrance in pyridine ring had no obvious effect on the outcome (Table 2, 8a–11a). Moreover, secondary amino pyridine (N-heptyl-2-aminopyridine) showed low reactivities in this catalyzed oxidative amidation (Table 2, 14a). To our delight, the methodology also worked well with aminopyrazine, aminopyrimidine and aminobenzothiazole (Table 2, 15a–18a) which further demonstrated the generality of the present approach.
Since reusability is one of the advantages for HPAILs catalyzed process, the potential recycling of HPAILs was investigated with the reaction of benzaldehyde 5a and 2-aminopyrine 6a under the optimized reaction conditions. After completion of the first reaction, the aqueous reaction mixture was concentrated under reduced pressure and then ethyl acetate was added. Upon vigorous stirring, the catalyst can be easily retrieved from the reaction mixture via simple centrifugation or filtration, washed with ethyl acetate to remove traces of the previous reaction mixture and then dried. The recovered catalyst was used for further runs for the same reaction. As was evident from Fig. 3, the reaction was repeated for up to five consecutive runs with a little loss of catalytic efficiency.
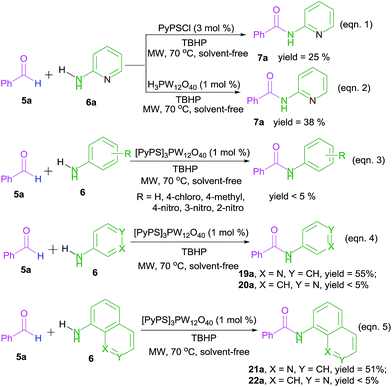
Furthermore, some control experiments were performed in order to investigate the reaction mechanism (Scheme 2). Firstly, when PyPSCl or pure H3PW12O40 was used as a catalyst under standard conditions, the relatively low yields implied the coordination of the PyPS cation and the PW12O40 anion (Scheme 2, eqn (1) and (2)). Secondly, several anilines (aniline, 4-chloroaniline, 4-methylaniline, 4-nitroaniline, 3-nitroaniline and 2-nitroaniline) were checked under optimized reaction conditions, but no desired products were formed (Scheme 2, eqn (3)). The results revealed that the heteroatom on the aromatic nucleus should be absolutely necessary for the oxidative amidation. In addition, in the cases of amino heterocycles with heteroatoms several bonds length distance from the reaction centre, 3-aminopyridine (Scheme 2, eqn (4), 19a) and 8-aminoquinoline (Scheme 2, eqn (5), 21a) gave moderate yields, while both 4-aminopyridine (Scheme 2, eqn (4), 20a) and 8-aminoisoquinoline (Scheme 2, eqn (5), 22a) as substrates resulted in very low yields, indicating that the nearness of heteroatoms on the aromatic nucleus with the reactive centre benefited the amidation.
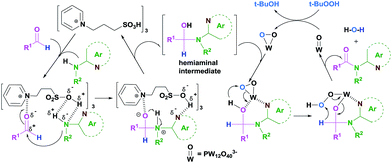
On the basis of the above analyses and our previous report,60 a N-directing dual-catalysis mechanistic pathway is proposed for understanding the catalytic performance of HPAILs in Fig. 4. Initially, the activation of carbonyl of aldehyde and N–H bond in amine are achieved via coordination with the aminium cation and sulfonic group in the HPAIL cation respectively. Subsequently, the addition of the amine to the carbonyl carbon atom give the dipolar adduct. After proton-exchange and desorption, a hemiaminal intermediate is formed with a regenerated HPAIL cation catalyst. Meanwhile, the oxidant reacts with the W sites in the HPAIL anion framework, leading to the generation of the peroxo-W complex that is supposed to be the active site which makes the hemiaminal oxidation. Upon the oxidation is directed and promoted by the coordination of heteroatoms on the aromatic nucleus to the W sites, the final amide product is obtained resuming the original state of W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O by ring opening, proton-exchange, subsequent dehydration and desorption of the adducted peroxo-W species.
O by ring opening, proton-exchange, subsequent dehydration and desorption of the adducted peroxo-W species.
Conclusions
In conclusion, all the above results demonstrate that an efficient and sustainable protocol for the synthesis of N-heteroaryl amides using HPAIL as catalyst and TBHP as an oxidant under MW-assisted and solvent-free conditions was achieved. Compared to previously known oxidative amidation catalysts, operational simplicity, solvent-free media, reusable catalysts and the compatibility with various functional groups are the advantages of this procedure. The proposed N-directing dual-catalysis process was briefly investigated. This report complements previous oxidative amidation strategy. On the basis of the findings of this report, the expansion of this chemistry to other organic transformations is currently underway in our laboratory.Experimental section
General methods
Reagent grade solvents were used for extraction, recrystallization and flash chromatography. All other commercial reagents were used as received without additional purification. The progress of reactions were checked by analytical thin-layer chromatography (TLC, silica gel 60 F-254 plates). The plates were visualized first with UV illumination followed by iodine or phosphomolybdic acid hydrate. Column chromatography was performed using silica gel (200–300 mesh). NMR spectra were obtained using BRUKER AVANCE III instrument. 1H NMR spectra were recorded at 300 MHz or 400 MHz and are reported in parts per million (ppm) on the δ scale relative to tetramethylsilane (TMS) as an internal standard. 13C NMR spectra were recorded at 75 MHz or 100 MHz and are reported in parts per million (ppm) on the δ scale relative to CDCl3 (δ 77.16) and DMSO-d6 (δ 39.52). Multiplicities are indicated as the following: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; dd, doubled doublet; td, tripled doublet; br, broad. Coupling constants (J values) where noted are quoted in Hertz. Mass spectra were obtained using Agilent 1260-6120 (ESI) instrument. MW-Promoted heating was obtained using MAS-II instrument manufactured by Shanghai Sineo Microwave Chemistry Technology Co., Ltd. The melting point was uncorrected.Typical procedure for the synthesis of N-heteroaryl amides by HPAILs catalyzed oxidative amidation
[PyPS]3PW12O40 (0.07 g, 0.02 mmol) and TBHP (0.39 g, 1.5 equiv., 70% aqueous solution) were added to a mixture of aldehyde (2 mmol) and amino heterocycle (2.4 mmol) in a 15 mL a glass pressure tube. After the pressure tube was closed, the reaction mixture was stirred at 70 °C under MW (700 W). The progress of the reaction was monitored by TLC. On completion, the aqueous reaction mixture was concentrated under reduced pressure, the remaining mixture was diluted with ethyl acetate (20 mL) with vigorous stirring for 30 min. The insoluble catalyst was recovered by filtration or centrifugation. After washing with ethyl acetate to remove traces of the previous reaction mixture and then drying, the recovered catalyst could be used for further runs for the same reaction. The filtrate was evaporated and the residue was directly purified by recrystallization or column chromatography to give amide product.Characterization data of N-heteroaryl amides
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was observed in the NMR spectra. Yellow solid. 1H NMR (300 MHz, CDCl3) δ 10.40 (brs, 1H), 10.21 (brs, 1H), 9.34 (d, J = 10.8 Hz, 1H), 8.32 (brs, 1H), 8.55 (s, 1H), 8.36–8.33 (m, 1H), 8.28 (d, J = 8.4 Hz, 1H), 7.76 (td, J = 8.0, 1.8 Hz, 1H), 7.69 (td, J = 7.8, 1.8 Hz, 1H), 7.137–7.06 (m, 2H), 6.96 (d, J = 8.1 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 163.2, 159.7, 151.2, 151.1, 148.6, 147.5, 139.0, 138.8, 120.3, 119.9, 115.3, 110.6; HRMS calcd for C6H7N2O (M + H+): 123.0553; found: 123.0556.
1) was observed in the NMR spectra. Yellow solid. 1H NMR (300 MHz, CDCl3) δ 10.40 (brs, 1H), 10.21 (brs, 1H), 9.34 (d, J = 10.8 Hz, 1H), 8.32 (brs, 1H), 8.55 (s, 1H), 8.36–8.33 (m, 1H), 8.28 (d, J = 8.4 Hz, 1H), 7.76 (td, J = 8.0, 1.8 Hz, 1H), 7.69 (td, J = 7.8, 1.8 Hz, 1H), 7.137–7.06 (m, 2H), 6.96 (d, J = 8.1 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 163.2, 159.7, 151.2, 151.1, 148.6, 147.5, 139.0, 138.8, 120.3, 119.9, 115.3, 110.6; HRMS calcd for C6H7N2O (M + H+): 123.0553; found: 123.0556.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was observed in the NMR spectra. White oil. 1H NMR (400 MHz, CDCl3) δ 8.13–8.11 (m, 4H), 7.91 (d, J = 4.4 Hz, 1H), 7.84 (d, J = 4.4 Hz, 1H), 7.52–7.35 (m, 8H), 6.62 (dd, J = 15.2, 8.8 Hz, 2H), 6.54 (t, J = 6.4 Hz, 1H), 6.46 (t, J = 6.4 Hz, 1H), 6.85–6.77 (m, 2H), 3.41–3.31 (m, 2H), 1.68–1.22 (m, 20H), 0.85–0.80 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 165.2, 157.4, 157.3, 142.9, 142.5, 140.3, 140.2, 134.3, 131.7, 129.8, 128.1, 111.5, 111.1, 108.1, 107.8, 53.5, 43.0, 42.0, 32.2, 32.1, 31.9, 29.4, 28.9, 27.4, 26.5, 22.7, 22.6, 14.2, 14.1; HRMS calcd for C19H25N2O (M + H+): 297.1961; found: 297.1963.
1) was observed in the NMR spectra. White oil. 1H NMR (400 MHz, CDCl3) δ 8.13–8.11 (m, 4H), 7.91 (d, J = 4.4 Hz, 1H), 7.84 (d, J = 4.4 Hz, 1H), 7.52–7.35 (m, 8H), 6.62 (dd, J = 15.2, 8.8 Hz, 2H), 6.54 (t, J = 6.4 Hz, 1H), 6.46 (t, J = 6.4 Hz, 1H), 6.85–6.77 (m, 2H), 3.41–3.31 (m, 2H), 1.68–1.22 (m, 20H), 0.85–0.80 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 165.2, 157.4, 157.3, 142.9, 142.5, 140.3, 140.2, 134.3, 131.7, 129.8, 128.1, 111.5, 111.1, 108.1, 107.8, 53.5, 43.0, 42.0, 32.2, 32.1, 31.9, 29.4, 28.9, 27.4, 26.5, 22.7, 22.6, 14.2, 14.1; HRMS calcd for C19H25N2O (M + H+): 297.1961; found: 297.1963.Acknowledgements
Financial Support from the National Natural Science Foundation of China (Grant No. 21302013), Suzhou Science and Technology Project (Grant No. SNG201620) and Natural Science Foundation of Jiangsu Province (Grant No. BK20161267 and BK20160405) are acknowledged.Notes and references
- J. W. Bode, Curr. Opin. Drug Discovery Dev., 2006, 9, 765–775 CAS.
- P. Kung, B. Huang, G. Zhang, J. Z. Zhou, J. Wang, J. A. Digits, J. Skaptason, S. Yamazaki, D. Neul, M. Zientek, J. Elleraas, P. Mehta, M. Yin, M. J. Hickey, K. S. Gajiwala, C. Rodgers, J. F. Davies and M. R. Gehring, J. Med. Chem., 2010, 53, 499–503 CrossRef CAS PubMed.
- T. W. J. Cooper, I. B. Campbell and S. J. F. Macdonald, Angew. Chem., 2010, 122, 8258–8267 (Angew. Chem., Int. Ed., 2010, 49, 8082–8091) CrossRef.
- C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC.
- G. Wang, T. Yuan and D. Li, Angew. Chem., 2011, 123, 1416–1419 (Angew. Chem., Int. Ed., 2011, 50, 1380–1383) CrossRef.
- H. Lundberg, F. Tinnis, N. Selander and H. Adolfsson, Chem. Soc. Rev., 2014, 42, 2714–2742 RSC.
- S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS PubMed.
- L. Ferrins, M. Gazdik, R. Rahmani, S. Varghese, M. L. Sykes, A. J. Jones, V. M. Avery, K. L. White, E. Ryan, S. A. Charman, M. Kaiser, C. A. S. Bergström and J. B. Baell, J. Med. Chem., 2014, 57, 6393–6402 CrossRef CAS PubMed.
- H. E. Colley, M. Muthana, S. J. Danson, L. V. Jackson, M. L. Brett, J. Harrison, S. F. Coole, D. P. Mason, L. R. Jennings, M. Wong, V. Tulasi, D. Norman, P. M. Lockey, L. Williams, A. G. Dossetter, E. J. Griffen and M. J. Thompson, J. Med. Chem., 2015, 58, 9309–9333 CrossRef CAS PubMed.
- C. Gao, B. J. Margolis, J. M. Strelow, L. R. Vidler and M. M. Mader, ACS Med. Chem. Lett., 2016, 7, 156–161 CrossRef CAS PubMed.
- S. C. Zimmermann, E. F. Wolf, A. Luu, A. G. Thomas, M. Stathis, B. Poore, C. Nguyen, A. Le, C. Rojas, B. S. Slusher and T. Tsukamoto, ACS Med. Chem. Lett., 2016, 7, 520–524 CrossRef CAS PubMed.
- S. Robert-Piessard, G. L. Baut, J. Courant, J. Brion, L. Sparfel, S. Bouhayat, J. Petit, R. Sanchez, M. Juge, N. Grimaud and L. Welin, Eur. J. Med. Chem., 1990, 25, 9–19 CrossRef CAS.
- J. M. Muñoz, J. Alcázar, A. Hoz, Á. Díaz-Ortiz and S. A. Diego, Green Chem., 2012, 14, 1335–1341 RSC.
- R. M. Lanigan, P. Starkov and T. D. Sheppard, J. Org. Chem., 2013, 78, 4512–4523 CrossRef CAS PubMed.
- L. Becerra-Figueroa, A. Ojeda-Porras and D. Gamba-Sánchez, J. Org. Chem., 2014, 79, 4544–4552 CrossRef CAS PubMed.
- V. Karaluka, R. M. Lanigan, P. M. Murray, M. Badland and T. D. Sheppard, Org. Biomol. Chem., 2015, 13, 10888–10894 CAS.
- M. Kumar, S. Bagchi and A. Sharma, New J. Chem., 2015, 39, 8329–8336 RSC.
- S. Rasheed, D. N. Rao, A. S. Reddy, R. Shankar and P. Das, RSC Adv., 2015, 5, 10567–10574 RSC.
- J. L. Vrijdag, F. Delgado, N. Alonso, W. M. D. Borggraeve, N. Pérez-Macias and J. Alcázar, Chem. Commun., 2014, 50, 15094–15097 RSC.
- Z. Yao and X. Wei, Chin. J. Chem., 2010, 28, 2260–2268 CrossRef CAS.
- S. M. Crawford, C. B. Lavery and M. Stradiotto, Chem.–Eur. J., 2013, 19, 16760–16771 CrossRef CAS PubMed.
- L. Nicolas, P. Angibaud, I. Stansfield, L. Meerpoel, S. Reymond and J. Cossy, RSC Adv., 2013, 3, 18787–18790 RSC.
- F. Karimi and B. Långström, Org. Biomol. Chem., 2003, 1, 541–546 CAS.
- Z. S. Qureshi, S. A. Revankar, M. V. Khedkar and B. M. Bhanage, Catal. Today, 2012, 198, 148–153 CrossRef CAS.
- C. Henry, D. Bolien, B. Ibanescu, S. Bloodworth, D. C. Harrowven, X. Zhang, A. Craven, H. F. Sneddon and R. J. Whitby, Eur. J. Org. Chem., 2015, 1491–1499 CrossRef CAS PubMed.
- L. Nahakpam, F. A. S. Chipem, B. S. Chingakham and W. S. Laitonjam, New J. Chem., 2015, 39, 2240–2247 RSC.
- P. Subramanian, S. Indu and K. P. Kaliappan, Org. Lett., 2014, 16, 6212–6215 CrossRef CAS PubMed.
- J. Du, K. Luo and X. Zhang, RSC Adv., 2014, 4, 54539–54546 RSC.
- W. Fan, Y. Yang, J. Lei, Q. Jiang and W. Zhou, J. Org. Chem., 2015, 80, 8782–8789 CrossRef CAS PubMed.
- S. Kumar, R. Vanjari, T. Guntreddi and K. N. Singh, RSC Adv., 2015, 5, 9920–9924 RSC.
- S. Yang, H. Yan, X. Ren, X. Shi, J. Li, Y. Wang and G. Huang, Tetrahedron, 2013, 69, 6431–6435 CrossRef CAS.
- Y. Ding, X. Zhang, D. Zhang, Y. Chen, Z. Wu, P. Wang, W. Xue, B. Song and S. Yang, Tetrahedron Lett., 2015, 56, 831–833 CrossRef CAS.
- H. N. Hareesh, K. U. Minchitha, N. Nagaraju and N. Kathyayini, Chin. J. Catal., 2015, 36, 1825–1836 CrossRef CAS.
- W. Dai, Y. Liu, T. Tong, X. Li and F. Luo, Chin. J. Catal., 2014, 35, 1012–1016 CrossRef CAS.
- O. P. S. Patel, D. Anand, R. K. Maurya and P. P. Yadav, Green Chem., 2015, 17, 3728–3732 RSC.
- B. W. Xin and J. C. Hao, Chem. Soc. Rev., 2014, 43, 7171–7187 RSC.
- K. V. Wagh and B. M. Bhanage, Green Chem., 2015, 17, 4446–4451 RSC.
- A. B. Bourlinos, K. Raman, R. Herrera, Q. Zhang, L. A. Archer and E. P. Giannelis, J. Am. Chem. Soc., 2004, 126, 15358–15359 CrossRef CAS PubMed.
- P. G. Rickert, M. R. Antonio, M. A. Firestone, K. A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, J. Phys. Chem. B, 2007, 111, 4685–4692 CrossRef CAS PubMed.
- P. G. Rickert, M. R. Antonio, M. Firestone, A. K. A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, Dalton Trans., 2007, 529–531 RSC.
- Y. Leng, J. Wang, D. Zhu, X. Ren, H. Ge and L. Shen, Angew. Chem., 2008, 121, 174–177 (Angew. Chem. Int. Ed., 2009, 48, 168–171) CrossRef.
- W. Zhang, Y. Leng, D. Zhu, Y. Wu and J. Wang, Catal. Commun., 2009, 11, 151–154 CrossRef CAS.
- H. Li, Y. Qiao, L. Hua, Z. Hou, B. Feng, Z. Pan, Y. Hu, X. Wang, X. Zhao and Y. Yu, ChemCatChem, 2010, 2, 1165–1170 CrossRef CAS.
- W. Zhang, Y. Leng, P. Zhao, J. Wang, D. Zhu and J. Huang, Green Chem., 2011, 13, 832–834 RSC.
- D. Fang, F. Wang, L. Wang, Y. Wu, J. Yang and C. Qian, Curr. Catal., 2012, 1, 197–201 CrossRef CAS.
- X. Zhang, D. Mao, Y. Leng, Y. Zhou and J. Wang, Catal. Lett., 2013, 143, 193–199 CrossRef CAS.
- Y. Leng, J. Wang, D. Zhu, M. Zhang, P. Zhao, Z. Long and J. Huang, Green Chem., 2011, 13, 1636–1639 RSC.
- Y. Leng, J. Wang, D. Zhu, L. Shen, P. Zhao and M. Zhang, Chem. Eng. J., 2011, 173, 620–626 CrossRef CAS.
- Y. Leng, W. Zhang, J. Wang and P. Jiang, Appl. Catal., A, 2012, 445–446, 306–311 CrossRef CAS.
- Y. Leng, J. Liu, C. Zhang and P. Jiang, Catal. Sci. Technol., 2014, 4, 997–1004 CAS.
- Z. Y. Long, Y. Zhou, G. J. Chen, W. L. Ge and J. Wang, Sci. Rep., 2014, 4, 3651–3655 CAS.
- Y. Leng, J. Liu, P. Jiang and J. Wang, ACS Sustainable Chem. Eng., 2015, 3, 170–176 CrossRef CAS.
- Y. Leng, J. Zhao, P. Jiang and J. Wang, RSC Adv., 2015, 5, 17709–17715 RSC.
- D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563–2591 CrossRef CAS PubMed.
- C. O. Kappe, Chem. Soc. Rev., 2008, 37, 1127–1139 RSC.
- V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629–639 CrossRef CAS PubMed.
- Z. Chen, R. Fu, W. Chai, H. Zheng, L. Sun, Q. Lu and R. Yuan, Tetrahedron, 2014, 70, 2237–2245 CrossRef CAS.
- R. Fu, Y. Yang, Z. Chen, W. Lai, Y. Ma, Q. Wang and R. Yuan, Tetrahedron, 2014, 70, 9492–9499 CrossRef CAS.
- R. Fu, Y. Yang, Y. Ma, F. Yang, J. Li, W. Chai, Q. Wang and R. Yuan, Tetrahedron Lett., 2015, 56, 4527–4531 CrossRef CAS.
- R. Fu, Y. Yang, Y. J. Zhang, J. Shao, X. Xia, Y. Ma and R. Yuan, Org. Biomol. Chem., 2016, 14, 1784–1793 CAS.
- Q. Wang, J. Feng, W. Chai, H. Geng, M. Xu, K. Wang, C. Xu, R. Fu and R. Yuan, Tetrahedron Lett., 2014, 55, 4785–4789 CrossRef CAS.
- Q. Wang, H. Zheng, W. Chai, D. Chen, X. Zeng, R. Fu and R. Yuan, Org. Biomol. Chem., 2014, 12, 6549–6553 CAS.
- Q. Wang, H. Geng, W. Chai, X. Zeng, M. Xu, C. Zhu, R. Fu and R. Yuan, Eur. J. Org. Chem., 2014, 6850–6853 CrossRef CAS.
- R. Fu, Y. Yang, W. Lai, Y. Ma, Z. Chen, J. Zhou, W. Chai, Q. Wang and R. Yuan, Synth. Commun., 2015, 45, 477–487 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of the 1H and 13C NMR spectra of all products. See DOI: 10.1039/c6ra20961k |
| This journal is © The Royal Society of Chemistry 2016 |