Two samarium(III) complexes with tunable fluorescence from in situ reactions of 2-ethoxy-6-((pyridin-2-ylmethylimino)methyl)phenol with Sm3+ ion†
Qin Wei,
Zhi-Peng Zheng*,
Hai-Xin Feng,
Xu-Jia Hong,
Xia Huang,
Hai-Jun Peng and
Yue-Peng Cai*
School of Chemistry and Environment, South China Normal University, Guangzhou Key Laboratory of Materials for Energy Conversion and Storage, Guangdong Provincial Engineering Technology Research Center for Materials for Energy Conversion and Storage, Guangzhou 510006, P. R. China. E-mail: ypcai8@yahoo.com; Fax: +86-020-39310187
First published on 29th September 2016
Abstract
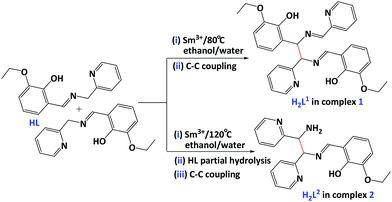
Two samarium(III) complexes showing different luminescent intensity, namely Sm(HL1)2·NO3·2H2O (1) and [SmL2(C2H5OH)2·NO3]2 (2), were constructed via the solvothermal in situ lanthanide metal–ligand reactions of 2-ethoxy-6-((pyridin-2-ylmethylimino)-methyl)phenol (HL) from pyridin-2-ylmethanamine and 3-ethoxy-2-hydroxybenzaldehyde with Sm3+ ion at different temperatures, in which H2L1 resulted from C–C coupling dimerization of HL, while H2L2 from C–C coupling of HL and pyridin-2-ylmethanamine.
The recently developed solvothermal in situ metal–ligand reaction might be a very effective means for preparation of complicated metal complexes,1 and the existence of –CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH2– moiety in some Schiff base ligands can result in in situ effective formation of the novel Schiff base ligands or aza-heterocycles, especially for the multi-arm podand-type ligands and complicated aza-heterocycles difficultly obtained from common organic synthetic reactions, such as inmidazo[1,5-a]pyridine, pyrazine, piperazine, imidazolidine and imidazole, as well as their derivatives from C–C/C–N coupling cycloaddition reactions of simple Schiff base ligands mediated by Cd2+, Zn2+, Fe2+, Co2+, Ni2+, Cu2+, Ti4+ etc.2 Compared to these widely studied in situ transition metal–ligand reactions, the corresponding in situ reactions induced by lanthanide metal ions have not yet been reported so far.
N–CH2– moiety in some Schiff base ligands can result in in situ effective formation of the novel Schiff base ligands or aza-heterocycles, especially for the multi-arm podand-type ligands and complicated aza-heterocycles difficultly obtained from common organic synthetic reactions, such as inmidazo[1,5-a]pyridine, pyrazine, piperazine, imidazolidine and imidazole, as well as their derivatives from C–C/C–N coupling cycloaddition reactions of simple Schiff base ligands mediated by Cd2+, Zn2+, Fe2+, Co2+, Ni2+, Cu2+, Ti4+ etc.2 Compared to these widely studied in situ transition metal–ligand reactions, the corresponding in situ reactions induced by lanthanide metal ions have not yet been reported so far.
On the other hand, the assembly of lanthanide(III) complexes has attracted much attention because of their interesting luminescent and magnetic properties.3 In order to obtain strongly luminescent lanthanide complexes, the selection and design of the organic ligand is very important, and it should be able to (i) efficiently absorb and transfer energy to the central metal ion and (ii) encapsulate and protect the lanthanide ion from the coordination of the solvent molecules.4 For these purposes, macrocyclic, macrobicyclic (cryptand) and podand type ligands containing aromatic rings are often good choices. In particular, podand ligands with many suitably designed arms could provide hosts for the lanthanide ions and well encapsulate them, by deliberate incorporation of appropriate multiple absorption groups (such as phenyl, benzimidazolyl, pyridyl groups, etc.) suitable for energy transfer. Therefore solvothermal in situ lanthanide metal–ligand reaction can provide a very good assembly strategy to prepare strongly luminescent lanthanide complexes.5
Our recent studies have focused on the use of a variety of salicylal/pyridyl-base Schiff base ligands for the synthesis of mono/poly-nuclear metal complexes.6 The research results show these simple Schiff base ligands can in situ convert into podand ligands with three/four suitably salicylal/pyridyl arms via transition metal-mediated C–C/C–N bond-forming strategy under solvothermal conditions. As part of our continuing studies focused on the solvothermal in situ metal–ligand reactions of simple Schiff base ligand, we report here two samarium(III) complexes Sm(HL1)2·NO3·2H2O (1) and [SmL2(C2H5OH)2·NO3]2 (2), which are obtained from the solvothermal in situ lanthanide metal–ligand reactions of simple Schiff base ligand of 2-ethoxy-6-((pyridin-2-yl-methylimino)methyl)phenol (HL, Scheme 1) with Sm3+ ion at different temperatures. Interestingly, in situ generated complex 1 has much better luminescent properties comparing with complex 2.
To the best of our knowledge, it is the first example of complexes formed from the solvothermal lanthanide metal–ligand reactions in Schiff base system with –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH2– moiety, in which two in situ generated novel multidentate Schiff base ligands of 6,6′-(5-phenyl-3-(pyridin-2-ylmethyl)-imidazolidine-2,4-diyl)bis(2-ethoxy-phenol)(H2L1), 2-((2-amino-1,2-di(pyridin-2-yl)-ethylimino)-methyl)-6-ethoxy-phenol(H2L2) (Scheme 1) were involved.
N–CH2– moiety, in which two in situ generated novel multidentate Schiff base ligands of 6,6′-(5-phenyl-3-(pyridin-2-ylmethyl)-imidazolidine-2,4-diyl)bis(2-ethoxy-phenol)(H2L1), 2-((2-amino-1,2-di(pyridin-2-yl)-ethylimino)-methyl)-6-ethoxy-phenol(H2L2) (Scheme 1) were involved.
As outlined in Scheme 1, the simple Schiff base ligand of 2-ethoxy-6-((pyridin-2-ylmethylimino)-methyl)phenol (HL) was synthesized in good yield by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 condensation of pyridin-2-ylmethanamine with 3-ethoxy-2-hydroxybenzaldehyde.6 Heating Sm(NO3)3·6H2O and HL (a 1
1 condensation of pyridin-2-ylmethanamine with 3-ethoxy-2-hydroxybenzaldehyde.6 Heating Sm(NO3)3·6H2O and HL (a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at molar ratio) in the mixed solvents of ethanol and water (a 3
2 at molar ratio) in the mixed solvents of ethanol and water (a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at volume) at 80 °C for 8 h afforded yellow block-like crystals Sm(HL1)2·NO3·2H2O (1) in good yield.‡ The products are all stable in air, and insoluble in water or common organic solvents. The formula of 1 was determined by single-crystal X-ray diffraction studies, elemental analysis (EA), infrared spectroscopy (IR) and thermogravimetric analysis (TGA).
1 ratio at volume) at 80 °C for 8 h afforded yellow block-like crystals Sm(HL1)2·NO3·2H2O (1) in good yield.‡ The products are all stable in air, and insoluble in water or common organic solvents. The formula of 1 was determined by single-crystal X-ray diffraction studies, elemental analysis (EA), infrared spectroscopy (IR) and thermogravimetric analysis (TGA).
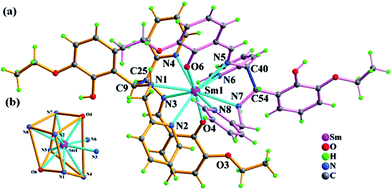
A single-crystal X-ray diffraction study performed on 1 reveals a neutral 0-D mononuclear compound.§ 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and each molecular unit contains one Sm3+ ion, two in situ formed (HL1)− ligands, one nitrate ion and two lattice water molecules. Each Sm(III) center in 1 has a ten-coordinated environment and adopts a distorted bicapped quadrangular prismatic coordination geometry with the N8O2 donor set of two η5-mode (HL1)− ligands as shown in Fig. 1. The Sm–N and Sm–O bond lengths range from 2.625(3) to 2.771(3) Å and from 2.372(3) to 2.398(2) Å (Table S1†), respectively. Intermolecular hydrogen bonding C(O)–H⋯O(N) (Table S2†) and π⋯π packing (the distances between centroids of two parallel pyridyl rings 4.077 Å) interactions result in the assembly of a 3-D supramolecular network in the ab plane (Fig. S1†).
and each molecular unit contains one Sm3+ ion, two in situ formed (HL1)− ligands, one nitrate ion and two lattice water molecules. Each Sm(III) center in 1 has a ten-coordinated environment and adopts a distorted bicapped quadrangular prismatic coordination geometry with the N8O2 donor set of two η5-mode (HL1)− ligands as shown in Fig. 1. The Sm–N and Sm–O bond lengths range from 2.625(3) to 2.771(3) Å and from 2.372(3) to 2.398(2) Å (Table S1†), respectively. Intermolecular hydrogen bonding C(O)–H⋯O(N) (Table S2†) and π⋯π packing (the distances between centroids of two parallel pyridyl rings 4.077 Å) interactions result in the assembly of a 3-D supramolecular network in the ab plane (Fig. S1†).
A solvothermal reaction of Sm(NO3)3·6H2O and HL (a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) in a mixed solvent of ethanol and water (a 1
2 molar ratio) in a mixed solvent of ethanol and water (a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at volume) at 120 °C for 8 h afforded yellow strip-like crystals [SmL2(C2H5OH)2·NO3]2 (2).‡ The products are all stable in air, and insoluble in water or common organic solvents. The formula of 2 was determined by single-crystal X-ray diffraction studies,§ elemental analysis (EA), infrared spectroscopy (IR) and thermogravimetric analysis (TGA).
1 ratio at volume) at 120 °C for 8 h afforded yellow strip-like crystals [SmL2(C2H5OH)2·NO3]2 (2).‡ The products are all stable in air, and insoluble in water or common organic solvents. The formula of 2 was determined by single-crystal X-ray diffraction studies,§ elemental analysis (EA), infrared spectroscopy (IR) and thermogravimetric analysis (TGA).
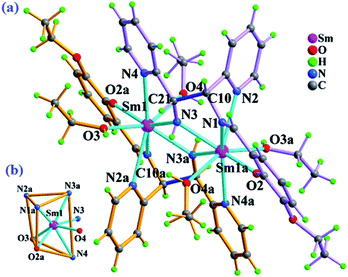
The crystal analysis result reveals that complex 2 crystallized in triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, and shows a discrete binuclear structure with a centrosymmetry, which comprised of two Sm3+ ions, two in situ formed (L2)2− ligands, four coordinated ethanol molecules and two free nitrate ions. In the asymmetric unit, Sm1 atom was octo-coordinated to two μ2-bridging amide nitrogen atoms (N3, N3a), two pyridine nitrogen atoms (N2a, N4), one imine nitrogen atom (N1a), one hydroxyl oxygen atom (O2a) and two ethanol oxygen atoms (O3, O4), showing a bicapped trigonal prismatic coordination geometry (Fig. 2). The Sm–N and Sm–O bond lengths range from 2.315(4) to 2.680(6) Å and from 2.226(4) to 2.471(5) Å (Table S1†), respectively. Intermolecular hydrogen bonding C(O)–H⋯O (Table S2†) interactions result in the assembly of a 2-D supramolecular layer in the ab plane (Fig. S2†).
space group, and shows a discrete binuclear structure with a centrosymmetry, which comprised of two Sm3+ ions, two in situ formed (L2)2− ligands, four coordinated ethanol molecules and two free nitrate ions. In the asymmetric unit, Sm1 atom was octo-coordinated to two μ2-bridging amide nitrogen atoms (N3, N3a), two pyridine nitrogen atoms (N2a, N4), one imine nitrogen atom (N1a), one hydroxyl oxygen atom (O2a) and two ethanol oxygen atoms (O3, O4), showing a bicapped trigonal prismatic coordination geometry (Fig. 2). The Sm–N and Sm–O bond lengths range from 2.315(4) to 2.680(6) Å and from 2.226(4) to 2.471(5) Å (Table S1†), respectively. Intermolecular hydrogen bonding C(O)–H⋯O (Table S2†) interactions result in the assembly of a 2-D supramolecular layer in the ab plane (Fig. S2†).
From above analyses and Scheme 1, the multidentate ligand H2L1 in compound 1 resulted from C–C coupling dimerization of HL. Different from H2L1, ligand H2L2 in 2 originated from C–C coupling of simple Schiff base ligand 2-ethoxy-6-((pyridin-2-ylmethylimino)-methyl)phenol (HL) and pyridin-2-ylmethanamine from partial hydrolysis of HL in solution state at high temperature (120 °C). Obviously, simple Schiff base HL in the assembly process of two compounds 1–2 underwent in situ C–C coupling reactions to form two Sm(III)-complexes 1 and 2 containing two new different ligands H2L1–2.
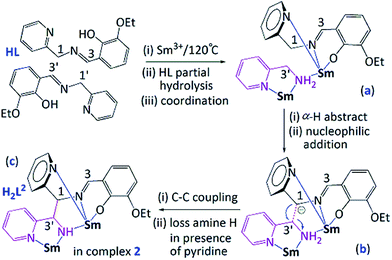
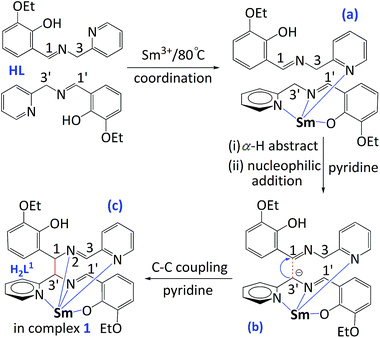
On the basis of the related literatures7 and our previous works,6 two possible mechanisms should be addressed in Schemes 2 and 3, respectively. At the stage of low temperature (80 °C), upon coordination to Sm(III) center via the imino nitrogen atom (the transition state (a) in Scheme 2) may result in a significantly increase of the activity of a C–H bond in the α-position to an imino group –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH–,8 pyridine, as the basic acceptor, has the ability to deprotonate the imino carbon-bound hydrogen atom to form carbanion.9 Such the methylene proton (α-H, H3′) was activated to produce a strong nucleophilic carbanion formed at C3′ (the transition state (b) in Scheme 2), and another molecule of H2L1 was drawn close by coordination bond between Sm(III) and pyridine nitrogen atom, then by nucleophilic attack of C3′ to C1, a C–C coupling reaction occurred to form the final product 1 containing ligand H2L1 ((c) in Scheme 2). In order to meet high coordination number of samarium ion (CN: 10), the resulting twisted molecular conformation of in situ formed H2L1 molecule prevented further occurrence of intramolecular C–C or C–N coupling reaction.
N–CH–,8 pyridine, as the basic acceptor, has the ability to deprotonate the imino carbon-bound hydrogen atom to form carbanion.9 Such the methylene proton (α-H, H3′) was activated to produce a strong nucleophilic carbanion formed at C3′ (the transition state (b) in Scheme 2), and another molecule of H2L1 was drawn close by coordination bond between Sm(III) and pyridine nitrogen atom, then by nucleophilic attack of C3′ to C1, a C–C coupling reaction occurred to form the final product 1 containing ligand H2L1 ((c) in Scheme 2). In order to meet high coordination number of samarium ion (CN: 10), the resulting twisted molecular conformation of in situ formed H2L1 molecule prevented further occurrence of intramolecular C–C or C–N coupling reaction.
 | ||
| Scheme 2 Proposed mechanism for the in situ formation of H2L1 shown in the asymmetric unit of complex 1. | ||
With the increase of temperature (120 °C), Schiff base ligand HL is easy to hydrolyze and the resulting product pyridin-2-ylmethanamine together with HL coordinated to Sm3+ ion to give intermediate (a) (Scheme 3). Similar to compound 1, coordination of the imino nitrogen atom to a metal Sm(III) center resulted in markedly increase of the acidity of a C–H bond in the α-position to an imino group –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH–, and then deprotonated the methylene proton (α-H1) in the presence of pyridine groups to produce a strong nucleophilic carbanion formed at C1 as intermediate (b) in Scheme 3. Subsequently, carbanion attacks the electrophilic carbon C3′ from a pyridin-2-ylmethanamine ligand to form C–C bond. After removal of amine hydrogen atoms in the existence of pyridine, the final complex 2 was formed ((c) in Scheme 3).
N–CH–, and then deprotonated the methylene proton (α-H1) in the presence of pyridine groups to produce a strong nucleophilic carbanion formed at C1 as intermediate (b) in Scheme 3. Subsequently, carbanion attacks the electrophilic carbon C3′ from a pyridin-2-ylmethanamine ligand to form C–C bond. After removal of amine hydrogen atoms in the existence of pyridine, the final complex 2 was formed ((c) in Scheme 3).
The phase purity of both 1 and 2 were supported by the powder X-ray diffraction (XRD) pattern of the bulk sample, which are consistent with the calculated patterns (Fig. S3†). TGA show that 1 loses 2.77% of its total weight upon heating to 153 °C, which corresponds to the loss of two lattice water molecules per formula unit (calcd 2.82%) and the molecular structure is stable up to 330 °C (Fig. S4†). While 2 loses 13.82% of its total weight upon heating to 155 °C, corresponding to four ethanol molecules per formula (calcd 13.84%). The TGA result shows that 2 is stable up to 385 °C.
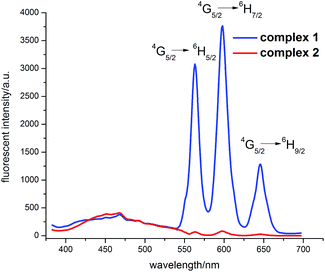
Solid-state luminescent properties of two complexes 1–2 are measured at room temperature. As can be seen from Fig. 3, when excited at 345 nm, complex 1 presents samarium-centered luminescence with its emission peak at 562 nm (4G5/2 → 6H5/2), 597 nm (4G5/2 → 6H7/2) and 643 nm (4G5/2 → 6H9/2), which is in good agreement with previous reported Sm3+ complexes.10 The most intense emission peak at 597 nm was monitored, and its lifetime was measured as 34.5 μs. According to the following equation, η = τ/τ0, where τ is the fluorescence lifetime (observed lifetime) of 4G5/2 excited state, and τ0 is the calculated natural lifetime (radiative lifetime) of 4G5/2 excited state,11 its quantum yield was calculated as 1.10%. While the ligand-centered emission at 468 nm is much weaker relative to that of Sm3+, indicating that the energy transfer from ligand to Sm3+ center is very efficient. However, samarium-centered luminescence is barely detected in complex 2, which could be probably ascribed to the existence of high energy O–H oscillators from four coordinated ethanol molecules, causing significant non-radiative decays of energy and resulting in luminescent quenching of 2 (details in Table S3 and Fig. S6†).12
In summary, through solvothermal in situ Sm–ligand (Schiff base HL) reaction, two samarium(III) complexes 1–2 are obtained at different temperature. The central Sm3+ ion in 1 was tightly encapsulated by two in situ generated ligands (HL1)−, well prevented from coordination of solvent molecules, therefore compound 1 shows very good Sm(III)-centered luminescent property. While in complex 2, the ligand (L2)2− failed to protect Sm3+ from coordination to ethanol molecules, because of which its Sm-based luminescence is quenched. Moreover, the possible formation mechanisms of two in situ generated ligands H2L1–2 are also carefully proposed. To the best of our knowledge, it is the first example of the solvothermal in situ C–C coupling reaction promoted by lanthanide metal ion in Schiff base system with –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–CH2– moiety. Obviously, this synthetic strategy provides a very effective approach for obtaining the multi-arm podand-type ligands and strongly lanthanide luminescent materials.
N–CH2– moiety. Obviously, this synthetic strategy provides a very effective approach for obtaining the multi-arm podand-type ligands and strongly lanthanide luminescent materials.
Acknowledgements
This work was supported by the national natural science foundation of P. R. China (Grant No. 21471061, 91122008 and 21671071), research fund for the doctoral program of higher education of China (Grant No. 20124407110007), Guangdong province higher school science and technology innovation key projects (Grant No. cxzd1113), Science and Technology Planning Project of Guangdong Province (Grant No. 2013B010403024 and 2015B010135009).Notes and references
- S. Hu, J.-C. Chen, M.-L. Tong, B. Wang, Y.-X. Yan and S. R. Batten, Angew. Chem., Int. Ed., 2005, 44, 5471 CrossRef CAS PubMed; S. M.-F. Lo, S. S.-Y. Chui, L.-Y. Shek, Z. Lin, X. X. Zhang, G.-H. Wen and I. D. Williams, J. Am. Chem. Soc., 2000, 122, 6293 CrossRef; Y.-J. Ou, Z.-P. Zheng, X.-J. Hong, L.-T. Wan, L.-M. Wei, X.-M. Lin and Y.-P. Cai, Cryst. Growth Des., 2014, 14, 5339 Search PubMed.
- S. Hu, J.-C. Chen, M.-L. Tong, B. Wang, Y.-X. Yan and S. R. Batten, Angew. Chem., Int. Ed., 2005, 117, 5607 CrossRef CAS; L. Han, W.-N. Zhao, Y. Zhou, X. Li and J.-G. Pan, Cryst. Growth Des., 2008, 8, 3504 Search PubMed; J. Carranza, C. Brennan, J. Sletten, J. M. Clemente-Juan, F. Lioret and M. Julve, Inorg. Chem., 2003, 42, 8716 CrossRef PubMed; B. A. Frazier, V. A. Villiams, P. T. Wolczanski, S. C. Bart, K. Meyer, T. R. Cundary and E. B. Lobkovsky, Inorg. Chem., 2013, 52, 3295 CrossRef PubMed; B. A. Frazier, P. T. Wolczanski, I. Keresztes, S. DeBeer, E. B. Lobkovsky, A. W. Pierpont and T. R. Cundari, Inorg. Chem., 2012, 51, 8177 CrossRef PubMed; B. A. Frazier, P. T. Wolczanski, E. B. Lobkovsky and T. R. Cundari, J. Am. Chem. Soc., 2009, 131, 3428 CrossRef PubMed.
- E. Pershagen, J. Nordholm and K. E. Borbas, J. Am. Chem. Soc., 2012, 134, 9832 CrossRef CAS PubMed; S. Comby, E. M. Surender, O. Kotova, L. K. Truman, J. K. Molloy and T. Gunnlaugsson, Inorg. Chem., 2014, 53, 1867 CrossRef PubMed; Z. Zhang, W.-X. Feng, P.-Y. Su, X.-Q. Lv, J.-R. Song, D.-D. Fan, W.-K. Wong, R. A. Jones and C.-Y. Su, Inorg. Chem., 2014, 53, 5950 CrossRef PubMed.
- N. Sabbatini, M. Guardigli and J. M. Lehn, Coord. Chem. Rev., 1993, 123, 201 CrossRef CAS; X.-P. Yang, C.-Y. Su, B.-S. Kang, X.-L. Feng, W.-L. Xiao and H.-Q. Liu, J. Chem. Soc., Dalton Trans., 2000, 3253 RSC.
- S. J. Bradberry, A. J. Savyasachi, M. Martinez-Calvo and T. Gunnlaugsson, Coord. Chem. Rev., 2014, 273–274, 226 CrossRef CAS; J.-M. Lehn and J.-B. Regnouf de Vains, Helv. Chim. Acta, 1992, 75, 1221 CrossRef; V. M. Mukkala and J. J. Kankare, Helv. Chim. Acta, 1992, 75, 1578 CrossRef; B. Alpha, J.-M. Lehn and G. Mathis, Angew. Chem., Int. Ed. Engl., 1987, 26, 266 CrossRef; V. Balzani, E. Berghmans, J.-M. Lehn, N. Sabbatini, A. Mecai, R. Therorde and R. Ziessel, Helv. Chim. Acta, 1990, 73, 2083 CrossRef.
- Z.-P. Zheng, Y.-J. Ou, X.-J. Hong, L.-M. Wei, L.-T. Wan, W.-H. Zhou, Q.-G. Zhan and Y.-P. Cai, Inorg. Chem., 2014, 53, 9625 CrossRef CAS PubMed; Z.-P. Zheng, Q. Wei, W.-X. Yin, L.-T. Wan, X. Huang, Y. Yu and Y.-P. Cai, RSC Adv., 2015, 5, 27682 RSC; Y.-J. Ou, Y.-J. Ding, Q. Wei, X.-J. Hong, Z.-P. Zheng, Y.-H. Long, Y.-P. Cai and X.-D. Yao, RSC Adv., 2015, 5, 27743 RSC.
- M. Roy, B. V. S. K. Chakravarthi, C. Jayabaskaran, A. A. Karande and A. R. Chakravarty, Dalton Trans., 2011, 40, 4855 RSC; M. E. Bluhm, M. Ciesielski, H. Görls, O. Walter and M. Döring, Inorg. Chem., 2003, 42, 8878 CrossRef CAS PubMed; D. H. Ess and K. N. Houk, J. Am. Chem. Soc., 2008, 130, 10187 CrossRef PubMed.
- V. M. Aryuzina and M. N. Shchukina, Khim. Geterotsikl. Soedin., 1968, 3, 506 Search PubMed; M. P. Kochergin and V. A. Lifanov, Khim. Geterotsikl. Soedin., 1994, 4, 490 Search PubMed; M. E. Bluhm, M. Görls, H. Ciesielski, O. Walter and M. Döring, Inorg. Chem., 2003, 42, 8878 CrossRef CAS PubMed.
- R. Huisgen, in 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, John Wiley & Sons, New York, 1984, vol. 1 Search PubMed.
- M. D. Regulacio, M. H. Pablico, J. A. Vasquez, P. N. Myers, S. Gentry, M. Prushan, S. Wah, T. Chang and S. L. Stoll, Inorg. Chem., 2008, 47, 1512 CrossRef CAS PubMed; H. S. Wang, B. Zhao, B. Zhai, W. Shi, P. Cheng, D. Z. Liao and S. P. Yan, Cryst. Growth Des., 2007, 7, 1851 Search PubMed; S. Quici, M. Cavazzini, G. Marzanni, G. Accorsi, N. Armaroli, B. Ventura and F. Barigelletti, Inorg. Chem., 2005, 44, 529 CrossRef PubMed.
- Z.-F. Li, J.-B. Yu, L. Zhou, H.-J. Zhang and R.-P. Deng, Inorg. Chem. Commun., 2008, 11, 1284 CrossRef CAS; X.-Y. Chen, M. P. Jensen and G.-K. Liu, J. Phys. Chem. B, 2005, 109, 13991 CrossRef PubMed.
- J.-C. G. Bünzli, Chem. Rev., 2010, 110, 2729 CrossRef CAS PubMed; S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189 RSC; Y.-W. Yip, H.-L. Wen, W.-T. Wong, P. A. Tanner and K.-L. Wong, Inorg. Chem., 2012, 51, 7013 CrossRef PubMed; D. Parker, Coord. Chem. Rev., 2000, 205, 109 CrossRef; X. J. Hong, M. F. Wang, H. G. Jin, Q. G. Zhan, Y. T. Liu, H. Y. Jia, X. Liu and Y. P. Cai, CrystEngComm, 2013, 15, 5606 RSC; M.-F. Wang, J.-Q. Su, H.-M. Peng, H.-Y. Jia, Q.-G. Zhan, H.-G. Jin, J.-H. Chen and Y.-P. Cai, Inorg. Chem. Commun., 2012, 21, 8 CrossRef.
- G. M. Sheldrick, SADABS, Version 2.05, University of Göttingen, Göttingen, Germany Search PubMed.
- G. M. Sheldrick, SHELXS-97, Program for X-ray Crystal Structure Determination, University of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of the synthetic procedures, spectra data and crystallographic data. CCDC 1061273 for 1 and 1061274 for 2. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra20957b |
| ‡ Preparation of 1A mixture of Sm(NO3)3·6H2O (17.78 mg, 0.04 mmol) and HL (40.96 mg, 0.16 mmol) was placed in a small vial containing ethanol (15 mL) and H2O (5 mL). The vial was sealed and heated at 80 °C for three day; yellow block-like crystals were collected, washed with ethanol and dried in air. Yield: 37.13 mg (73% based on Sm). Elemental analysis (%) calcd for C60H66N9O13Sm (Mr = 1271.56): C 56.62, H 5.19, N 9.91. Found: C 56.57, H 5.24, N 9.95. IR (KBr, cm−1): 3415(br, vs), 2981(w), 2893(w), 1631(s), 1603(m), 1556(m), 1488(m), 1460(m), 1426(w), 1389(m), 1275(m), 1246(w), 1216(s), 1110(w), 1046(s), 992(w), 904(w), 842(w), 785(m), 739(m), 663(m), 557(w), 469(m).Preparation of 2A mixture of Sm(NO3)3·6H2O (35.56 mg, 0.08 mmol) and HL (40.96 mg, 0.16 mmol) was placed in a Teflon-lined stainless steel autoclave containing ethanol (10 mL) and H2O (10 mL), and heated to 120 °C for 72 h. After the mixture was cooled to room temperature at the rate of 10 °C h−1, pale yellow crystals of 2 were collected, washed with water and dried at room temperature, yield: 34.58 mg (65% based on Sm). Elemental analysis (%) calcd for C50H64N10O14Sm2 (Mr = 1329.81): C 45.12, H 4.81, N 10.53. Found: C 45.17, H 4.83, N 10.52. IR (KBr, cm−1): 3479(br, vs), 3305(w), 2971(w), 1636(vs), 1559(vs), 1421(m), 1414(s), 1305(w), 1281(w), 1235(w), 1210(s), 1150(w), 1079(m), 1040(m), 907(w), 769(m), 744(m), 645(m), 521(w), 442(w). |
§ Crystallographic data for 1M = 1271.56, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.4150(18) Å, b = 15.719(2) Å, c = 16.661(2) Å, α = 97.375(2)°, β = 103.260(2)°, γ = 105.382(2)°, V = 2988.8(7) Å3, Z = 2, μ = 1.053 mm−1, Dc = 1.413 Mg m−3, F(000) = 1310, 6278 unique (Rint = 0.0495), R1 = 0.0709, wR2 = 0.1617[I > 2σ(I)], GOF = 1.031. The intensity data were collected on a Bruker Smart Apex II CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 298 K. All absorption corrections were performed by using the SADABS program.13 Structural solutions and full-matrix least-squares refinements based on F2 were performed with the SHELXS-97 (ref. 14) and SHELXL-2014/7 (ref. 15) program packages, respectively. All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms on organic ligand was generated by the riding mode (C–H = 0.93 or 0.96 Å), and the water hydrogen atoms were located from difference maps and refined with isotropic temperature factors. Two oxygen (O10, O11) atoms of nitrate in 1 are disordered into two positions, and each position has a site occupancy factor of 0.5.Crystallographic data for 2M = 1329.81, triclinic, space group P , a = 12.4150(18) Å, b = 15.719(2) Å, c = 16.661(2) Å, α = 97.375(2)°, β = 103.260(2)°, γ = 105.382(2)°, V = 2988.8(7) Å3, Z = 2, μ = 1.053 mm−1, Dc = 1.413 Mg m−3, F(000) = 1310, 6278 unique (Rint = 0.0495), R1 = 0.0709, wR2 = 0.1617[I > 2σ(I)], GOF = 1.031. The intensity data were collected on a Bruker Smart Apex II CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 298 K. All absorption corrections were performed by using the SADABS program.13 Structural solutions and full-matrix least-squares refinements based on F2 were performed with the SHELXS-97 (ref. 14) and SHELXL-2014/7 (ref. 15) program packages, respectively. All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms on organic ligand was generated by the riding mode (C–H = 0.93 or 0.96 Å), and the water hydrogen atoms were located from difference maps and refined with isotropic temperature factors. Two oxygen (O10, O11) atoms of nitrate in 1 are disordered into two positions, and each position has a site occupancy factor of 0.5.Crystallographic data for 2M = 1329.81, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 10.718(4) Å, b = 11.181(4) Å, c = 13.217(5) Å, α = 79.809(5)°, β = 81.813(5)°, γ = 65.165(4)°, V = 1408.3(9) Å3, Z = 1, μ = 2.135 mm−1, Dc = 1.568 Mg m−3, F(000) = 670, 8348 unique (Rint = 0.0247), R1 = 0.0439, wR2 = 0.0880[I > 2σ(I)], GOF = 1.037. The intensity data were collected on a Bruker Smart Apex II CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 298 K. All absorption corrections were performed by using the SADABS program.13 Structural solutions and full-matrix least-squares refinements based on F2 were performed with the SHELXS-97 (ref. 14) and SHELXL-2014/7 (ref. 15) program packages, respectively. All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms on organic ligand was generated by the riding mode (C–H = 0.93 or 0.96 Å). , a = 10.718(4) Å, b = 11.181(4) Å, c = 13.217(5) Å, α = 79.809(5)°, β = 81.813(5)°, γ = 65.165(4)°, V = 1408.3(9) Å3, Z = 1, μ = 2.135 mm−1, Dc = 1.568 Mg m−3, F(000) = 670, 8348 unique (Rint = 0.0247), R1 = 0.0439, wR2 = 0.0880[I > 2σ(I)], GOF = 1.037. The intensity data were collected on a Bruker Smart Apex II CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 298 K. All absorption corrections were performed by using the SADABS program.13 Structural solutions and full-matrix least-squares refinements based on F2 were performed with the SHELXS-97 (ref. 14) and SHELXL-2014/7 (ref. 15) program packages, respectively. All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms on organic ligand was generated by the riding mode (C–H = 0.93 or 0.96 Å). |
| This journal is © The Royal Society of Chemistry 2016 |