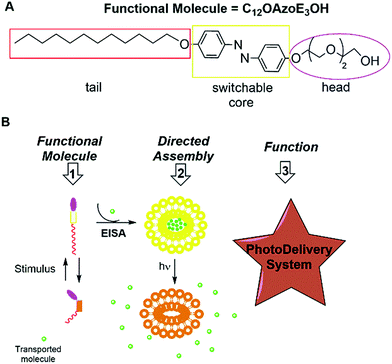
Easy directed assembly of only nonionic azoamphiphile builds up functional azovesicles†
M. A. Sequeira,
M. G. Herrera,
Z. B. Quirolo and
V. I. Dodero‡
 *
*
Instituto de Química del Sur (INQUISUR-CONICET), Departamento de Química, Universidad Nacional del Sur, 8000FTN Bahía Blanca, Argentina. E-mail: veronica.dodero@uns.edu.ar
First published on 8th November 2016
Abstract
We obtained functional azovesicles based only on a simple nonionic azoamphiphile, C12OazoE3OH. The process combines evaporation-induced and solvent-induced directed assembly.
Directed assembly has emerged as a powerful strategy to obtain functional systems under non-equilibrium conditions controlled by the processing conditions.1 In this context, aromatic rod-coil small molecules with amphiphilic features can be very useful because their self-assembly in water is accomplished by thermodynamically driven separation between the polar medium and the rigid π-surface. On the other hand, as π–π interactions are directional such self-assembly can produce structurally precise nano-structures from small molecules.2 In general, the easy way to obtain advanced materials is evaporation – induced self-assembly (EISA).3 Typically, it consists in the controlled evaporation of the solvent under rotatory evaporation, allowing solute concentration and the formation of a thin organized mesostructure.3a,b Once the thin film is hydrated at the transition temperature the formation of nano-objects in water might occur. By this methodology, vesicular systems such as liposomes and niosomes are built up.4 A further step is to obtain stimuli responsive vesicles; in this context azobenzene E–Z photoisomerization emerges as a simple molecular spatio-temporal switch which undergoes a large geometric change and dipole moment depending on light conditions.5 Previously, an ammonium azobenzene derivative was mixed with a commercial amphiphile obtaining different multi-state nanostructures depending on light conditions.6 Recently, other ammonium azobenzene amphiphile was mixed with cholesterol sulphate obtaining non-phospholipid fluid liposomes.7 Nonionic azoamphiphiles are seldom investigated; mainly because nonionic amphiphiles are known to form ill-defined aggregates in water.8 However, two pure azoamphiphiles with nonionic hydroxyl head form stable nano-objects in water by spontaneous self-assembly.9 As far as we are concerned, there are no reports of directed assembly of pure small azobenzene amphiphiles in water. Even less with nonionic features, although they are excellent candidates to form robust nano-objects in water. The major advantage is that the uncharged head allows that the tails conformation change due to photoisomerization is transferred efficiently to the whole aggregate.9 Recently, we have obtained a synthetically simple and versatile photoswitchable nonionic azoamphiphile, 4-dodecyloxi-4′-(1-hydroxytriethylenglycol) azobenzene, C12OazoE3OH (Scheme 1A).10,§ The interfacial behaviour of C12OazoE3OH alone and among a model biomembrane was evaluated by Langmuir and Gibbs monolayers. We have proved that C12OazoE3OH might be an efficient photo-responsible probe to remote control of biological membrane properties.10
Here, with respect to previous paper, we point out that C12OazoE3OH develops functional pure azovesicles without the assistance of surfactants or lipids by EISA (Scheme 1B).
Our hypothesis is based on some relevant features of C12OazoE3OH. First, the length of head would result in an increase in the minimum aggregation number at which pure vesicles become geometrically allowed.11 Second, the C12 hydrophobic tail would favor bilayer formation.11 Third, C12OazoE3OH develops a Smetic C mesophase, which was confirmed recently by RDX.12 Finally, at the interface pure C12OazoE3OH compressive modulus (bending elasticity) decrease from 100 mN m−1 (E) to circa 60 mN m−1 upon E–Z transformation.10 In other words, the change in the effective cross-sectional area of C12OazoE3OH due to photoisomerization led to a substantial change of the interfacial properties and probably this change occurs in bulk, too.11,9b
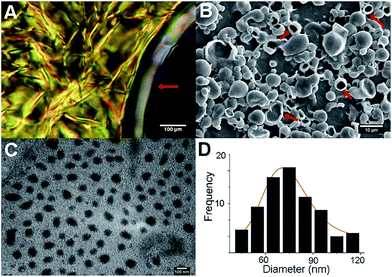
We envisaged that directing the assembly of C12OazoE3OH into liquid crystalline state,13 followed by hydration of the film at the transition temperature will build up pure azovesicles in water. To check our working hypothesis, the lyotropic behaviour of C12OazoE3OH in chloroform was tested by Lawrence's penetration experiment.14 This experiment consists in melting the solid between two coverslips obtaining a homogenous film. Then, chloroform was allowed to penetrate the solid by capillarity and polarized optical microscopy (POM) observation showed the sequentially obtained mesophases. The result is a fast phase diagram at room temperature, showing the formation of a periodic hexagonal, cubic and lamellar mesophase on increasing concentration (Fig. 1A).3a
The lamellar mesophase of C12OazoE3OH was confirmed after film evaporation at room temperature from a 19 mM solution followed by POM observation (see ESI†). As many aromatic amphiphiles, C12OazoE3OH is not soluble in water at room temperature, however the critical aggregation concentration (c.a.c) was determinated below 0.35 μM at 70 °C (Krafft point) (further details see ESI†).
Next, we performed EISA3 from a chloroform solution of C12OazoE3OH,§ using a round bottom flask and a rotary evaporator (30 °C). By this methodology, as the liquid flows away from the contact line it would result in the uniform solute deposition on the substrate. Subsequent hydration at Krafft temperature (70 °C) followed by shaking and ageing for two days led to stable giant vesicles of pure C12OazoE3OH of 1 to 10 μm diameter, as detected by microscopic techniques (Fig. 1B; further details see ESI†).
Mild sonication led to nanovesicles of 73 ± 1 nm, detected by transmission electron microscopy (Fig. 1C and D see ESI†).
The molecular organization of the azobenzene in the vesicles and the efficiency of photoisomerization in the aggregate state were evaluated by simple UV/Vis absorption spectroscopy. In general, azobenzenes are prone to form J-aggregates and H-aggregates, which possess characteristic UV-Vis spectra.15 In water, the two bands corresponding to the π–π* transition of (E) C12OazoE3OH were observable; one at 260 nm, and the second at 311 nm (Fig. 2A). In chloroform, the second band was observed at 358 nm.10 The blue-shifted of the band from 358 to 311 nm is indicative of strong interaction of the chromophores and is characteristic of H-aggregates in bilayers and cylindrical micelles.15 Spherical micelles are not able to form H aggregates because the packing is too loose and the chromophores cannot adopt the required configuration in the micelle.15 This supports the formation of nano-(E) azovesicles and it is well correlated with the morphology observed by TEM. After UV-light illumination a hypochromic shift of both bands at 260 nm and 311 nm occurs, showing that after photoisomerization the chromophores were still as H-aggregates, the photostationary state (pss) was E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z (46
Z (46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54), for simplicity we will refer to this state as (Z) azovesicles. The new (Z) azovesicles are less rigid than the (E) azovesicles; because of that, TEM observation was not suitable to determinate their size (ESI†). Photo-reversion was achieved after irradiation with a white light bulb (140 min 60 watts).
54), for simplicity we will refer to this state as (Z) azovesicles. The new (Z) azovesicles are less rigid than the (E) azovesicles; because of that, TEM observation was not suitable to determinate their size (ESI†). Photo-reversion was achieved after irradiation with a white light bulb (140 min 60 watts).
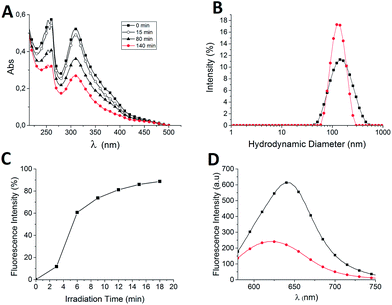 | ||
Fig. 2 (A) UV-Vis spectra of C12OazoE3OH under UV-light illumination in water (0.5 mM, 150 watts). (B) DLS measurements of hydrodynamic diameter of C12OazoE3OH before ( ) and after ( ) and after ( ) UV-light illumination in water (140 min, 150 watts). (C) Calcein release during the E–Z isomerization at 310 nm of C12OazoE3OH vesicles (excitation 415 nm and emission at 515 nm, 150 watts). Passive release was less than 10% after two days. (D) Nile red spectra of C12OazoE3OH system before ( ) UV-light illumination in water (140 min, 150 watts). (C) Calcein release during the E–Z isomerization at 310 nm of C12OazoE3OH vesicles (excitation 415 nm and emission at 515 nm, 150 watts). Passive release was less than 10% after two days. (D) Nile red spectra of C12OazoE3OH system before ( ) and after ( ) and after ( ) UV-light illumination (12 min, 150 watts). Nile Red contribution in the same experimental conditions was subtracted. For experimental details, please refer to ESI.† ) UV-light illumination (12 min, 150 watts). Nile Red contribution in the same experimental conditions was subtracted. For experimental details, please refer to ESI.† | ||
The photomodulation of the (E) azovesicles was further supported by particle size measurements from dynamic light scattering (Fig. 2B). Initially, the hydrodynamic diameter of (E) azovesicles were 166 ± 79 nm and after UV-light irradiation a contraction of the system occurs with a final diameter of 136 ± 41 nm. It seems that the change of the effective cross-sectional area of the C12OazoE3OH, due to E–Z photoisomerization promoted a change in the size/shape of the azovesicles.9b,11,16
To prove the photo-delivery properties of C12OazoE3OH nanovesicles, an encapsulation experiment with the hydrophilic fluorescent dye, calcein is presented. To this aim, the mentioned EISA protocol was performed but, during the hydration step, a solution of 100 mM of calcein in HEPES buffer was employed.17 After size exclusion chromatography, the free calcein was separated from the calcein-loaded (E) azovesicles.¶ Photoisomerization experiments were performed using the spectrofluorometer (310 nm, 6 cycles of 3 minutes irradiation each). An increase of calcein fluorescence at 515 nm was observed depending on irradiation time (Fig. 2C). The maximum release was 97% considering the maximal release of calcein-loaded (E) azovesicles upon Triton X-100 treatment (ESI†).17 To further investigate the nature of the azovesicles, the hydrophobic Nile red dye (NR) was added to a dispersion of (E) azovesicles. Nile red senses microenvironment changes by a large blueshift of the emission λmax depending on probe microenvironment (108 nm from water to hexane) and it is well used to stain neutral lipids in cells. Additionally, NR fluorescence intensity decreased in polar media and/or in the presence of hydrophilic environment.18
Upon binding to the preformed (E) azovesicles, NR emission was blueshifted from λmax 658 nm to 643 nm (Fig. 2D and ESI†). After E–Z photoisomerization, NR emission was blueshifted again to λmax 615 nm indicating that NR is in more hydrophobic environment. Moreover, the emission spectrum after E–Z isomerization was broad indicating different binding modes. The decrease of the fluorescence intensity after E–Z photoisomerization has been previously explained considering the increase of dipolar moment of the Z isomer.19 Furthermore; it shows that, after irradiation the system is more fluid allowing the NR dye to penetrate better into the superstructure with the consequence blueshift of the emission wavelength. The observed behavior is in good agreement with the aforementioned decrease of the bending elasticity at the interface11 and justify the calcein release upon UV-light illumination.
In conclusion, we develop functional azovesicles or azoniosomes obtained from a small molecule by simple EISA procedures. Taking advantage of the simplicity of the protocols, this strategy might open new opportunities to build up advanced materials from pure pi-conjugated amphiphilic small molecules.
Acknowledgements
This work was supported by UNS-PGI (Universidad Nacional del Sur), CONICET (National Scientific and Technical Research Council) and ANCyPT PICT-PRH 2010–2013 (National Agency for Promotion of Science and Technology) Argentinian Research grants. M. A. S., M. G. H., Z. B. Q. are also grateful for their CONICET fellowship. The authors thank Dr L. Benedini for technical support in c.a.c evaluation. V. I. D. acknowledges her Alexander von Humboldt Foundation fellowship (HERMES).Notes and references
- (a) J. L. Jiménez Blanco, et.al., Chem. Commun., 2016, 52, 10117 RSC; (b) A. Nikoubashman, V. E. Lee, C. Sosa, R. K. Prud’homme, R. D. Priestley and A. Z. Panagiotopoulos, ACS Nano, 2016, 10, 1425 CrossRef CAS PubMed; (c) M. D. Kim, S. A. Derguno and E. Pinkhassik, Langmuir, 2015, 31, 2561 CrossRef PubMed.
- (a) J.-H. Ryu, D.-J. Hong and M. Lee, Chem. Commun., 2008, 1043 RSC; (b) M. Molla and S. Ghosh, Phys. Chem. Chem. Phys., 2014, 16, 26672 RSC; (c) M. J. Hollamby, et al., Nat. Chem., 2014, 6, 690 CrossRef CAS PubMed.
- (a) C. J. Brinker, Y. Lu, A. Sellinger and H. Fan, Adv. Mater., 1999, 11, 579 CrossRef CAS; (b) D. Grosso, F. Cagnol, G. J. d. A. A. Soler-Illia, E. L. Crepaldi, H. Amenitsch, A. Brunet-Bruneau, A. Bourgeois and C. Sanchez, Adv. Funct. Mater., 2004, 14, 309 CrossRef CAS; (c) W. Han and Z. Lin, Angew. Chem., Int. Ed., 2012, 51, 1534 CrossRef CAS PubMed.
- (a) F. M. Menger and K. Gabrielson, Angew. Chem., Int. Ed., 1995, 34, 2091 CrossRef CAS; (b) P. Walde, H. Umakoshi, P. Stano and F. Mavelli, Chem. Commun., 2014, 50, 10177 RSC; (c) S. Gupta, R. Tyagi, V. Parmar, S. Sharma and R. Haag, Polymer, 2012, 53, 3053 CrossRef CAS; (d) E. Soussan, S. Cassel, M. Blanzat and I. Rico-Lattes, Angew. Chem., Int. Ed., 2009, 48, 274 CrossRef CAS PubMed.
- B. Feringa, Molecular Switches, ed. B. Feringa, Wiley-VCH, Verlag GmbH, 2001, p. 399 Search PubMed.
- Y. Wang, N. Ma, Z. Wang and X. Zhang, Soft Matter, 2010, 6, 902 RSC.
- Z.-K. Cui, T. Phoeung, P.-A. Rousseau, G. Rydzek, Q. Zhang, C. G. Bazuin and M. Lafleur, Langmuir, 2014, 30, 10818 CrossRef CAS PubMed.
- (a) C. Tanford in The Hydrophobic Effect, Wiley, New York, 1973 Search PubMed; (b) R. Nagarajan and E. Ruckenstein, J. Colloid Interface Sci., 1979, 71, 580 CrossRef CAS.
- (a) C. Kördel, C. Popeney and R. Haag, Chem. Commun., 2011, 47, 6584 RSC; (b) T. Shang, K. Smith and T. Hatton, Langmuir, 2006, 22, 1436 CrossRef CAS PubMed.
- L. A. Benedini, M. A. Sequeira, M. L. Fanani, B. Maggio and V. I. Dodero, J. Phys. Chem. B, 2016, 120, 4053 CrossRef CAS PubMed.
- (a) J. Israelachvili, J. Mitchell and B. Ninham, Biochim. Biophys. Acta, Biomembr., 1977, 470, 185 CrossRef CAS; (b) T. Kunitake and Y. Okahata, J. Am. Chem. Soc., 1977, 99, 3860 CrossRef CAS.
- X. Tan, R. Zhang, C. Guo, X. Cheng, H. Gao, F. Liu, J. R. Bruckner, F. Giesselmann, M. Prehm and C. Tschierske, J. Mater. Chem. C, 2015, 3, 11202 RSC.
- M. Lee and Y.-S. Yoo, J. Mater. Chem., 2002, 12, 2161 RSC.
- E. S. Blackmore and G. J. T. Tiddy, J. Chem. Soc., Faraday Trans. 2, 1988, 84, 1115 RSC.
- (a) X. Song, J. Perlstein and D. Whitten, J. Am. Chem. Soc., 1997, 119, 9144 CrossRef CAS; (b) J. Kuiper and J. Engberts, Langmuir, 2004, 20, 1152 CrossRef CAS PubMed; (c) R. A. Moss and W. Jiang, Langmuir, 1995, 11, 4217 CrossRef CAS; (d) J. Kuiper, M. Stuart and J. Engberts, Langmuir, 2008, 24, 426 CrossRef CAS PubMed.
- (a) Y. Orihara, A. Matsumura, Y. Saito, N. Ogawa, T. Saji, A. Yamaguchi, H. Sakai and M. Abe, Langmuir, 2001, 17, 6072 CrossRef CAS; (b) A. Pulido-Companys, R. Albalat, J. Garcia-Amoros, D. Velasco and J. Ignes-Mullol, Langmuir, 2013, 29, 9635 CrossRef CAS PubMed.
- (a) C. L. Ávila, et al., J. Biol. Chem., 2014, 289, 13838 CrossRef PubMed; (b) S. L. Huang and R. C. MacDonald, Biochim. Biophys. Acta, 2004, 1665, 134 CrossRef CAS PubMed.
- (a) P. Greenspan, E. P. Mayer and S. D. Fowler, J. Cell Biol., 1985, 100, 965 CrossRef CAS PubMed; (b) F. Deye, T. A. Berger and A. G. Anderson, Anal. Chem., 1990, 62, 615 CrossRef; (c) M. G. Herrera, T. V. Veuthey and V. I. Dodero, Colloids Surf., B, 2016, 141, 565 CrossRef CAS PubMed.
- (a) M. Kumari, M. Billamboz, E. Leonard, C. Len, C. Böttcher, A. K. Prasad, R. Haag and S. K. Sharma, RSC Adv., 2015, 5, 48301 RSC; (b) T. Yuan, J. Dong, G. Hana and G. Wang, RSC Adv., 2016, 6, 10904 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and additional figures. See DOI: 10.1039/c6ra20933e |
| ‡ Present Address: Chemistry Department, Bielefeld University, Germany. E-mail; E-mail: veronica.dodero@uni-bielefeld.de |
§ In CHCl3 solution, pure C12OazoE3OH exists as photostationary state (pss) of E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z (95 Z (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) which, upon UV-light illumination, photoisomerizes in one minute to a second pss E 5) which, upon UV-light illumination, photoisomerizes in one minute to a second pss E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z (10 Z (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90). Complete physical and spectroscopic characterization is in ref. 10. 90). Complete physical and spectroscopic characterization is in ref. 10. |
| ¶ Passive release was less than 10% after two days in darkness. |
| This journal is © The Royal Society of Chemistry 2016 |