Facile preparation of CoNi2S4@NiSe nano arrays on compressed nickel foam for high performance flexible supercapacitors†
Zhonghua Tang,
Chunyang Jia*,
Zhongquan Wan,
Qianlong Zhou,
Xingke Ye and
Yucan Zhu
State Key Laboratory of Electronic Thin Films and Integrated Devices, School of Microelectronics and Solid-State Electronics, University of Electronic Science and Technology of China, Chengdu 610054, P. R. China. E-mail: cyjia@uestc.edu.cn; Fax: +86 28 83202569; Tel: +86 28 83201991
First published on 21st November 2016
Abstract
CoNi2S4@NiSe nano arrays with hierarchical structure are in situ prepared on compressed nickel foam for a high performance flexible supercapacitor by a three-step solution-based method which involves a one-step solvothermal process and a two-step hydrothermal process. During the preparation process, the nickel foam serves not only as a support but also as the nickel source for NiSe nanorods, which is beneficial for improving the electronic conductivity and reducing the contact resistant of the active materials/current collector. The CoNi2S4@NiSe nano arrays with ample active sites and porous structure contribute to increasing the electrochemical performance. The flexible supercapacitor based on CoNi2S4@NiSe nano arrays was prepared, which exhibits a high areal capacitance of 312.95 mF cm−2 at a scan rate of 5 mV s−1 and good cycling stability with 97.59% capacitance retention after 1000 cycles. Meanwhile, it also exhibits excellent electrochemical stability which can maintain great capacitive performance even in the bending state. Therefore, the CoNi2S4@NiSe nano arrays with hierarchical structure on the compressed nickel foam could be a promising electrode material for high performance flexible supercapacitors.
1. Introduction
In recent years, with rapidly increasing demands for high performance and flexible electronic devices, flexible energy storage technology has attracted great interest.1–4 Supercapacitors, also known as ultra-capacitors, have received considerable attention due to their high power density, low maintenance costs, fast charge and discharge processes, and so forth.5–11 According to the charge storage mechanism, the supercapacitors are divided into two different types: electrical double-layer supercapacitors (electrostatic energy storage mechanism) and pseudocapacitors (redox energy storage mechanism).12 Due to the limited specific capacitance and energy density of electrical double-layer supercapacitors, pseudocapacitors have been attracted lots of interests.8,13–16Ni-Based materials, such as NiO,17 Ni(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18,19 and NiS,20–23 have been widely investigated as the electrode materials for supercapacitors owning to their low cost and nontoxic than RuO2.24,25 Attributed to the valence electronic configuration of Ni (3d84s2) and the small difference in electronegativity between Ni (χ = 1.9) and Se (χ = 2.4), Ni and Se can form a variety of complexes with varied stoichiometries26 with multiple oxide states, which indicate that they're beneficial for being the electrode materials for pseudocapacitor.27 Compared with the conductive oxide nanostructures such as TiO2,28 SnO2
18,19 and NiS,20–23 have been widely investigated as the electrode materials for supercapacitors owning to their low cost and nontoxic than RuO2.24,25 Attributed to the valence electronic configuration of Ni (3d84s2) and the small difference in electronegativity between Ni (χ = 1.9) and Se (χ = 2.4), Ni and Se can form a variety of complexes with varied stoichiometries26 with multiple oxide states, which indicate that they're beneficial for being the electrode materials for pseudocapacitor.27 Compared with the conductive oxide nanostructures such as TiO2,28 SnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 and ZnO30 nanorod arrays as the core materials, the NiSe nano arrays as the cores not only can provide effective electron transport path but also can contribute to capacitance.27 In addition, the NiSe nanorod is believed as good electrode material for pseudocapacitor, it not only has multiple oxide states, but also can in situ grow on nickel foam with the nickel foam as nickel source, and the electrode materials exhibit excellent kinetics of charges and ions with short diffusion paths.27 However, as the single-metal-component (Ni-based) materials, NiSe nanorod has relative low conductive activity and single morphology compared to the binary-metal-component.31–36 CoNi2S4, as one of binary-metal-component materials with easy synthesis, high conductivity, ample active sites and abundant redox reactions,37–39 can in situ grow on NiSe nanorods with specific morphology. It is believed that decorating NiSe nanorods with CoNi2S4 will benefit for preparing the high performance electrode materials for pseudocapacitors.
29 and ZnO30 nanorod arrays as the core materials, the NiSe nano arrays as the cores not only can provide effective electron transport path but also can contribute to capacitance.27 In addition, the NiSe nanorod is believed as good electrode material for pseudocapacitor, it not only has multiple oxide states, but also can in situ grow on nickel foam with the nickel foam as nickel source, and the electrode materials exhibit excellent kinetics of charges and ions with short diffusion paths.27 However, as the single-metal-component (Ni-based) materials, NiSe nanorod has relative low conductive activity and single morphology compared to the binary-metal-component.31–36 CoNi2S4, as one of binary-metal-component materials with easy synthesis, high conductivity, ample active sites and abundant redox reactions,37–39 can in situ grow on NiSe nanorods with specific morphology. It is believed that decorating NiSe nanorods with CoNi2S4 will benefit for preparing the high performance electrode materials for pseudocapacitors.
In this paper, with the aim of designing and fabricating high performance electrode materials for flexible supercapacitors, CoNi2S4@NiSe nano arrays in situ grown on the compressed nickel foam with hierarchical structure were synthesized. During the synthesis process, the nickel foam serves not only as a support but also as the nickel source for NiSe nanorods, CoNi2S4 nanosheets were further in situ grown on the NiSe nanorods. CoNi2S4@NiSe nano arrays with hierarchical structure have multiple oxide states and ample redox reactions to realize high performance energy storage. The electrode of CoNi2S4@NiSe nano arrays with abundant active sites and porous structure provide abundant ion transmission paths. In addition, the nickel foam maintains its flexibility after growing active materials, owing to the press process before active materials was grown on it. The electrochemical properties of CoNi2S4@NiSe nano arrays on compressed nickel foam were further studied as electrode materials for flexible supercapacitors.
2. Materials and experimental section
2.1 Materials
Selenium powder, ethylene glycol, ethanediamine, Ni(NO3)2·6H2O, Co(NO3)2·6H2O, Na2S·9H2O, nickel foam and hexamethylenetetramine (HMT) were purchased from Sigma-Aldrich. All chemicals were analytical grade, used as received without any further purification, and their aqueous solutions were prepared with deionized water.2.2 Experimental
In the next step, 45.34 mg Na2S·9H2O was dissolved with 75 mL deionized water in the beaker, with continuous stirring at room temperature for 30 min, then the solution was transferred into a clean Teflon-lined stainless steel autoclave with the volume as 100 mL. NiSe@nickel foam with Ni–Co precursor was immersed in the reaction solution and maintained 120 °C for 8 h. After the Teflon-lined stainless steel autoclave cooled to room temperature, the novel 3D hierarchical structure of CoNi2S4@NiSe nano arrays electrode materials were prepared. After washing with ethanol and deionized water alternatively several times, the samples were dried at room temperature naturally. The mass loading of CoNi2S4@NiSe nano arrays is about 0.866 mg by subtracting the amount of nickel foam from the total of CoNi2S4@NiSe@nickel foam.
2.3 Materials characterization
The morphologies and structures of these samples were characterized by scanning electron microcopy (SEM, JEOL JSM-7600F), high resolution transmission electron microscopy (HRTEM, JEOL 2100F) and X-ray diffraction (XRD, SHIMADZU XRD-7000) by using Cu Kα (λ = 0.15406 nm) radiation. All electrochemical tests were carried out on a electrochemical workstation (CHI660E, Chenhua, Shanghai), including cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS). Cycling performance of present supercapacitor based on GCD were tested in Land battery testing system (CT2001A). All the cells areas were approximate to 1 cm × 1 cm.Areal specific capacitance is concluded from the CV curves based on eqn (1), the gravimetric specific capacitance is calculated from the CV curves based on eqn (2), the gravimetric specific capacitance is calculated from the GCD curves based on eqn (3). ED and PD are the energy and power density of the supercapacitor, which have been calculated as shown in the eqn (4) and (5), respectively.
 | (1) |
 | (2) |
 | (3) |
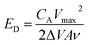 | (4) |
 | (5) |
In these equations, CA is the areal specific capacitance (F cm−2), I is the constant discharging current (A), and the value of  is equal to the area of the CV curves, A is the total area of the electrode material (cm2), Δt is the discharge time (s), ν is the scan rate (V s−1), ΔV is the potential window during the charge and discharge process (V), Cm is the gravimetric specific capacitance (F g−1), m is the mass of active materials (g), Vmax is the operation potential (V).
is equal to the area of the CV curves, A is the total area of the electrode material (cm2), Δt is the discharge time (s), ν is the scan rate (V s−1), ΔV is the potential window during the charge and discharge process (V), Cm is the gravimetric specific capacitance (F g−1), m is the mass of active materials (g), Vmax is the operation potential (V).
3. Results and discussion
Fig. 2 shows the procedure for preparation of CoNi2S4@NiSe@nickel foam electrode materials. Solvothermal and hydrothermal techniques were utilized to fabricate the composite electrode. First, the NiSe nanorods were grown on the compressed nickel foam, and the nickel foam serves not only as a support but also as the nickel source for NiSe nanorods. Then, the CoNi2S4 nanosheets were in situ grown on the NiSe nanorods. In order to maintain the flexible of nickel foam and improve the electrochemical performance of the electrode, nickel foam was pressed before the active materials was grown on it.Fig. 3 shows the difference between the pressed nickel foam and unpressed one. Compared with the unpressed nickel foam,40–45 electrode based on the pressed one is efficient in keeping its flexibility and improving the electrochemical performance, we can get the reasons as follow: in terms of flexibility, the pressed nickel foam become more compact, and more reactions happened on the surface of nickel foam, which reduce the inner reaction of nickel foam to maintain its flexibility (Fig. 3a and b); in terms of electrochemical performance, due to the electrochemical performance of supercapacitor is mainly decided by the active materials on the surface of substrate, and the pressed nickel foam make more reactions occurred on the substrate surface than the unpressed one, so that its performance was improved (Fig. 3c and d). Thus, the electrode based on the pressed nickel foam is efficient in keeping its flexibility and improving the electrochemical performance.
3.1 Morphology and structure characterization
The well-defined electrode materials of CoNi2S4@NiSe@nickel foam with hierarchical structure was verified by XRD (Fig. 4), SEM and HRTEM (Fig. 6). As shown in Fig. 4a, the crystal structure of NiSe was verified, which is made up of hexagonal NiSe phase (JCPDS 02-0892, 2θ = 29.8°, 32.8°, 44.4°, 49.9°, 59.7°, 61.2°, 69.1° attributed to the (100), (101), (102), (110), (103), (201) and (202) planes) (Fig. 4a). Then, the crystal structure of CoNi2S4 was verified (JCPDS 24-0334, 2θ = 31.4°, 38.4°, 47.2°, 50.4°, 68.8° belongs to the (311), (400), (422), (511) and (444) planes) (Fig. 4b), and the strong peaks marked with asterisks (Fig. 4a and b) were originated from nickel foam. The XPS spectra are shown in Fig. 5. Ni 2p3/2 and Ni 2p1/2 peaks appeared at 855.4 and 873.2 eV, respectively (Fig. 5a). The peak at 852.9 eV was corresponding to metallic Ni 2p, which came from unreacted nickel substrate. These peaks have two shake-up satellites at 861.7 and 880.2 eV, which indicated that Ni was in the Ni2+ oxidation state. In Fig. 5b, one pair of binding energies at 780.0 and 796.2 eV correspond to Co 2p3/2 and Co 2p1/2, respectively. Another pair appeared in the higher energy region around 781.4 and 801.2 eV. Two kinds of cobalt oxidation state: Co2+ and Co3+ can be confirmed from the two pairs of doublet which are characteristic of Co 2p and Co 3p.46 As shown in Fig. 5c, two main peaks and one shake-up satellite are presented in the S 2p spectrum. The binding energy at 162.5 eV is typical of metal–sulfur bonds in the ternary metal sulfides, while the binding energy at 169.9 eV can be attributed to the sulfur ions with higher oxide state of S4O62− at the surface owing to partly oxidation of CoNi2S4.47 The Se 3d features of NiSe shown in Fig. 5d, and the peak at 68.7 eV can be assigned to SeSx.27 These results were in good agreement with the XRD pattern. Through the SEM, we can see that NiSe was grown on nickel foam uniformly (Fig. 6a), and the NiSe nanorods (70–100 nm in diameter and several micrometers in length) were grown around the skeleton of nickel foam (Fig. 6b). Meanwhile, these nanorods were made up of many smaller nanorods (Fig. 6c), such as a channel was composed of many paths, which benefited for ion and charge transmission. Not only that, and CoNi2S4 nanosheets (several nanometers in thickness) were in situ growth on the NiSe nanorods (Fig. 6d–f), which will further add the active sites to improve the kinetics of charges and ions in the electrode. Fig. 6g presents the HRTEM images of an individual NiSe nanorod, it reveals that the NiSe has a lattice fringe with interplane spacing of 0.232 nm, corresponding to plane of NiSe. The HRTEM image of CoNi2S4@NiSe@nickel foam reveals that the CoNi2S4 nanosheets were grown around the NiSe nanorod (Fig. 6h). These results demonstrate that the CoNi2S4 nanosheets were successfully in situ growth on the NiSe nanorods, which is advantage of charges and ions transporting.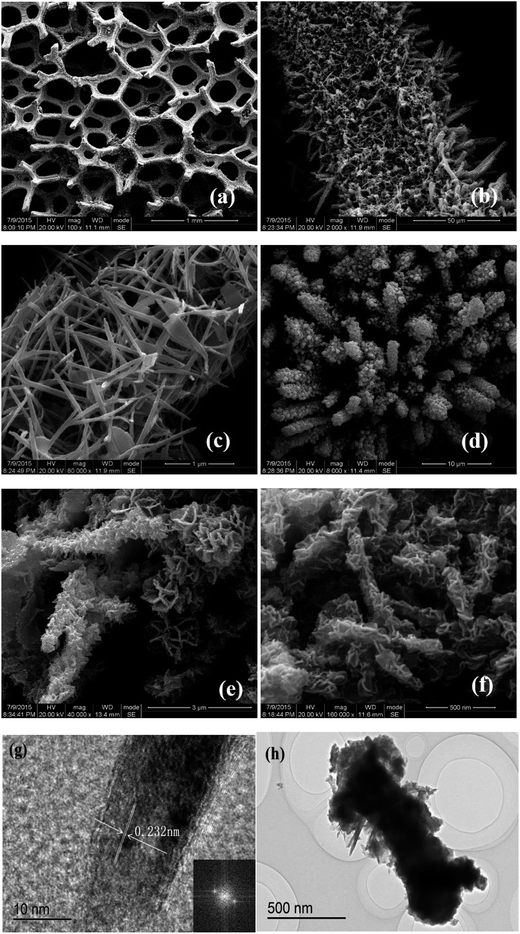 | ||
| Fig. 6 SEM images of (a–c) NiSe@nickel foam and (d–f) CoNi2S4@NiSe@nickel foam, HRTEM images of (g) NiSe@nickel foam and (h) CoNi2S4@NiSe@nickel foam electrode materials. | ||
3.2 Electrochemical measurements
To demonstrate the electrochemical performance benefits of CoNi2S4@NiSe@nickel foam, the symmetry flexible supercapacitor based on CoNi2S4@NiSe@nickel foam was tested by CV tests, GCD tests and EIS tests (Fig. 7a, d and f), and the same tests have been done on the flexible supercapacitor based on NiSe@nickel foam for comparison (Fig. 7b, e and g).Fig. 7a and b show the typical CV curves of flexible supercapacitors with the potential window from 0 to 0.8 V at various scan rates ranging from 5 to 50 mV s−1. The flexible supercapacitor based on CoNi2S4@NiSe@nickel foam show a higher specific capacitance than that of flexible supercapacitor based on NiSe@nickel foam, which is attributed to the CoNi2S4 nanosheets layer (Fig. 7c). CoNi2S4 nanosheets not only increase the active sites of the electrode materials, but also increase the kinds of redox reactions, thus resulting in improved capacitive performance. The possible reversible faradaic redox reactions during the cathodic and anodic sweeps between the NiSe@nickel foam and electrolyte, as well as between the CoNi2S4@NiSe@nickel foam and electrolyte were presented. Since the sulfur is in the same family with oxygen, the redox reaction of mechanism of the CoNi2S4 is similar to the CoNi2O4 in the alkaline electrolyte,48–50 which can be described as the following equations:
Accordingly the redox reaction of nickel sulfide, cobalt sulfide and nickel selenide with OH− can be illustrated as follows:51–53
It was clear that these well-defined redox peaks can be explained as related with reversible faradaic reactions of MX/MXOH and MXOH/MXO (M = Ni and Co, X = S and Se). With increasing the sweep rates, the anodic peak current density increases and the cathodic peak current density decreases, which suggests that a relatively low resistance of the electrode and fast redox reactions at the interface of the electrode and electrolyte.34
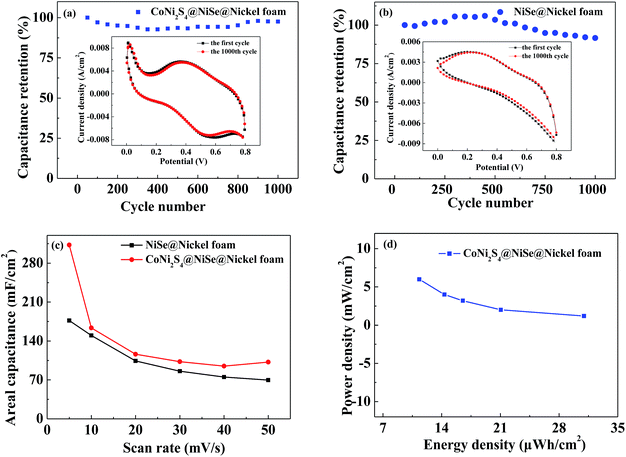
GCD tests within a potential window from 0 to 0.8 V were carried out for further evaluating the capacitive performance at the current densities of 3, 5, 8, 10 and 15 mA cm−2 (Fig. 7d and e). The specific capacitance of the supercapacitor was calculated via the eqn (3). These results exhibit that the calculated specific capacitance decreases with the current density increasing, and capacitance values of flexible supercapacitor based on CoNi2S4@NiSe@nickel foam can get as high as 311.25, 216.88, 180, 156.25 and 136 mF cm−2 at the applied current densities of 3, 5, 8, 10 and 15 mA cm−2. These values are in agreement with capacitance values of CV curves and higher than that of flexible supercapacitor based on NiSe@nickel foam. The enhanced electrochemical performance could be attributed to the multiple oxide states and abundant redox reactions in the binary-metal composite as well as the ample active sites in the hierarchical structure.
To further evaluate the electrochemical behaviors of the flexible supercapacitors, the EIS measurements were carried out at a frequency ranging from 0.01 to 100 kHz. And the corresponding Nyquist plots of two supercapacitors are shown in Fig. 7f. In the low-frequency region, both of the flexible supercapacitors exhibit a more vertical line leaning to imaginary axis more than 45°, suggesting the more facile electrolyte ions diffusion to the active material and more ideal capacitor behavior, which further confirms that the electrode can remain good capacitive performances54–56 In the high-frequency region, the internal resistance (Rs), including electrolyte resistance, active materials ohmic resistance and active materials/current collector' contact resistant, can be read from the intersection of the high-frequency part at the real axis of the pattern.57 The Rs of flexible supercapacitors based CoNi2S4@NiSe@nickel foam is 0.5764 Ω, and that of flexible supercapacitors based on NiSe@nickel foam is 0.3743 Ω (Fig. 7f inset one). The favorable low value of Rs may be attributed to the conductive substrate and the in situ growth of the active materials on the compressed nickel foam. In the intermediate frequency region, there is a negligible semicircle, indicating a particularly low charge transfer resistance. According to the comparison image of the EIS (Fig. 7f), in the high-frequency region, no distinct semicircle shape is observed, demonstrating the fast charge transfer behaviour inside the electrode during its charge–discharge process, which benefit from the hierarchical structure. In the low-frequency region, the straight line of flexible supercapacitor based on CoNi2S4@NiSe@nickel foam lean more toward the imaginary axis, indicating the lower diffusion resistance. The reduced Faraday resistance rendered by the multiple oxide states and abundant redox reactions in the intriguing material combination and the ample active sites in the hierarchical structure lead to enhanced electrochemical reaction.
In addition, the cycling life and specific capacitance retention of the flexible supercapacitors are shown in Fig. 8a and b. Flexible supercapacitor based on CoNi2S4@NiSe@nickel foam (Fig. 8a) can maintain 97.59% and flexible supercapacitor based on NiSe@nickel foam (Fig. 8b) can keep 89.73% after 1000 cycles of CV at the scan rate of 50 mV s−1. Areal specific capacitance vs. scan rates is plotted (Fig. 8c), the areal specific capacitance of flexible supercapacitors based on CoNi2S4@NiSe@nickel foam is 312.95 mF cm−2, the gravimetric specific capacitance is 1686.028 F g−1 and the energy density is 30.97 μW h cm−2 at the scan rate of 5 mV s−1. These values not only are much higher than those of the single component electrode of NiSe but also conventional supercapacitors.37 Accordingly, the areal specific capacitance of flexible supercapacitors based on NiSe@nickel foam is 176.75 mF cm−2, the gravimetric specific capacitance is 851.912 F g−1 and the energy density is 9 μW h cm−2 at the scan rate of 5 mV s−1. The cycling performance of supercapacitor based on CoNi2S4@NiSe@nickel foam by the test of galvanostatic charge–discharge has been presented in Fig. S1.† Specific energy and specific power are the two key factors for evaluating capacitance of the flexible supercapacitors. Fig. 8d is the Ragone plot (power density vs. energy density) of flexible supercapacitor based on CoNi2S4@NiSe@nickel foam, which shows relatively good performance. Through the above results, we can know the flexible supercapacitor based on CoNi2S4@NiSe nano arrays with ample active sites and porous structure, which provides abundant ion transmission paths to enhanced the electrochemical performance. In addition, multiple oxide states and abundant redox reactions in the electrode of CoNi2S4@NiSe with hierarchical structure also contribute to increasing the electrochemical performance. The electrochemical performance for CoNi2S4@NiSe@nickel foam in this study and some reported Ni-based compounds electrodes has been presented in Table S1† for comparison.
In order to evaluate the flexibility of the device, we have test its capacitance retention in bending state. Fig. 9 is the CV curves of flexible supercapacitor based on CoNi2S4@NiSe@nickel foam in bending state. Compared to the device without bending, the capacitance performance of the device in the bending state is slightly improved, which can be explained as follows: in the bending state, the distance of electrode will be shorter than the without bending, which is more beneficial for the ion moving in the electrolyte to enhance the specific capacitance. This result confirms the highly flexible nature of the CoNi2S4@NiSe@nickel foam electrode.
 | ||
| Fig. 9 Capacitance retention of flexible supercapacitor based on CoNi2S4@NiSe@nickel foam with and without bending. | ||
4. Conclusions
In summary, we combined solvothermal and hydrothermal methods to fabricate hierarchical structure of CoNi2S4@NiSe@nickel foam nano arrays for high performance flexible supercapacitor. The NiSe nanorods and CoNi2S4 nanosheets provide abundant active sites, which are beneficial for the kinetics of charges and ions in the electrode. The capacitive performance of flexible supercapacitor based on CoNi2S4@NiSe@nickel foam was superior to that of the flexible supercapacitor based on NiSe@nickel foam at all scan rates and discharge current densities. The newly synthesized electrode material CoNi2S4@NiSe@nickel foam with hierarchical structure also exhibits a high areal capacitance, a high cycling stability and excellent electrochemical stability. The flexible electrode based on nickel foam can be got by pressing nickel foam before the active material was grown, which provide a method to fabricate flexible supercapacitors.Acknowledgements
We thank the National Natural Science Foundation of China (Grant No. 21572030, 21272033, 21402023) and Technology Innovative Research Team of Sichuan Province of China (No. 2015TD0005) for financial support.References
- D. Kim, G. Lee, D. Kim and J. S. Ha, ACS Appl. Mater. Interfaces, 2015, 7, 4608–4615 CAS.
- L. F. Chen, Z. H. Huang, H. W. Liang, W.-T. Yao, Z. Y. Yu and S. H. Yu, Energy Environ. Sci., 2013, 6, 3331–3338 CAS.
- Y. Liu, B. Weng, J. M. Razal, Q. Xu, C. Zhao, Y. Hou, S. Seyedin, R. Jalili, G. G. Wallace and J. Chen, Sci. Rep., 2015, 5, 17045 CrossRef CAS PubMed.
- J. Chen, C. Jia and Z. Wan, Electrochim. Acta, 2014, 121, 49–56 CrossRef CAS.
- K. Wang, W. Zou, B. Quan, A. Yu, H. Wu, P. Jiang and Z. Wei, Adv. Energy Mater., 2011, 1, 1068–1072 CrossRef CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- Q. Zhou, X. Ye, Z. Wan and C. Jia, J. Power Sources, 2015, 296, 186–196 CrossRef CAS.
- J. Yan, Q. Wang, T. Wei and Z. Fan, Adv. Energy Mater., 2014, 4, 1300816 CrossRef.
- J. Chen, C. Jia and Z. Wan, Synth. Met., 2014, 189, 69–76 CrossRef CAS.
- B. E. Conway and W. G. Pell, J. Solid State Electrochem., 2003, 7, 637–644 CrossRef CAS.
- D. Cai, D. Wang, C. Wang, B. Liu, L. Wang, Y. Liu, Q. Li and T. Wang, Electrochim. Acta, 2015, 151, 35–41 CrossRef CAS.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- J. H. Kim, K. Zhu, Y. Yan, C. L. Perkins and A. J. Frank, Nano Lett., 2010, 10, 4099–4104 CrossRef CAS PubMed.
- D. Duc Tai, X. Wang and J. M. Lee, Nano Energy, 2013, 2, 1303–1313 CrossRef.
- D. Ghosh, M. Mandal and C. K. Das, Langmuir, 2015, 31, 7835–7843 CrossRef CAS PubMed.
- Z. Tang, C. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272–1278 CrossRef CAS.
- Y. Li, K. Ye, K. Cheng, J. Yin, D. Cao and G. Wang, J. Power Sources, 2015, 274, 943–950 CrossRef CAS.
- C. Sun, M. Ma, J. Yang, Y. Zhang, P. Chen, W. Huang and X. Dong, Sci. Rep., 2014, 4, 7054 CrossRef CAS PubMed.
- W. Wei, L. Mi, Y. Gao, Z. Zheng, W. Chen and X. Guan, Chem. Mater., 2014, 26, 3418–3426 CrossRef CAS.
- J. Yang, W. Guo, D. Li, C. Wei, H. Fan, L. Wu and W. Zheng, J. Power Sources, 2014, 268, 113–120 CrossRef CAS.
- J. Xu, K. Wang, S. Z. Zu, B. H. Han and Z. Wei, ACS Nano, 2010, 4, 5019–5026 CrossRef CAS PubMed.
- W. Chen, C. Xia and H. N. Alshareef, ACS Nano, 2014, 8, 9531–9541 CrossRef CAS PubMed.
- X. Yu, W. Sun and Y. Chu, New J. Chem., 2014, 38, 70–76 RSC.
- C. Tang, Z. Pu, Q. Liu, A. M. Asiri, X. Sun, Y. Luo and Y. He, ChemElectroChem, 2015, 2, 1903–1907 CrossRef CAS.
- S. Yang, K. Cheng, J. Huang, K. Ye, Y. Xu, D. Cao, X. Wang and G. Zhang, Electrochim. Acta, 2014, 120, 416–422 CrossRef.
- W. Xu, K. Zhao, C. Niu, L. Zhang, Z. Cai, C. Han, L. He, T. Shen, M. Yan, L. Qu and L. Mai, Nano Energy, 2014, 8, 196–204 CrossRef CAS.
- X. Li, Z. Wang, Y. Qiu, Q. Pan and P. Hu, J. Alloys Compd., 2015, 620, 31–37 CrossRef CAS.
- H. Chen, J. Jiang, L. Zhang, D. Xia, Y. Zhao, D. Guo, T. Qi and H. Wan, J. Power Sources, 2014, 254, 249–257 CrossRef CAS.
- H. Wan, J. Liu, Y. Ruan, L. Lv, L. Peng, X. Ji, L. Miao and J. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 15840–15847 CAS.
- J. Zhang, H. Gao, M. Y. Zhang, Q. Yang and H. X. Chuo, Appl. Surf. Sci., 2015, 349, 870–875 CrossRef CAS.
- J. Yang, M. Ma, C. Sun, Y. Zhang, W. Huang and X. Dong, J. Mater. Chem. A, 2015, 3, 1258–1264 CAS.
- Y. Zhu, Z. Wu, M. Jing, X. Yang, W. Song and X. Ji, J. Power Sources, 2015, 273, 584–590 CrossRef CAS.
- L. Shen, L. Yu, H. B. Wu, X.-Y. Yu, X. Zhang and X. W. Lou, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- J. Tang, J. Shen, N. Li and M. Ye, Ceram. Int., 2015, 41, 6203–6211 CrossRef CAS.
- L. Chen, Y. Zhou, H. Dai, T. Yu, J. Liu and Z. Zou, Nano Energy, 2015, 11, 697–703 CrossRef CAS.
- N. Kurra, C. Xia, M. N. Hedhili and H. N. Alshareef, Chem. Commun., 2015, 51, 10494–10497 RSC.
- D. Cai, H. Huang, D. Wang, B. Liu, L. Wang, Y. Liu, Q. Li and T. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 15905–15912 CAS.
- C. Tang, N. Cheng, Z. Pu, W. Xing and X. Sun, Angew. Chem., Int. Ed., 2015, 54, 9351–9355 CrossRef CAS PubMed.
- W. L. Yang, Z. Gao, J. Ma, J. Wang, B. Wang and L. H. Liu, Electrochim. Acta, 2013, 112, 378–385 CrossRef CAS.
- X. Xia, J. Tu, Y. Mai, X. Wang, C. Gu and X. Zhao, J. Mater. Chem., 2011, 21, 9319–9325 RSC.
- Y. Gao, S. Chen, D. Cao, G. Wang and J. Yin, J. Power Sources, 2010, 195, 1757–1760 CrossRef CAS.
- R. Zou, M. F. Yuen, L. Yu, J. Hu, C. S. Lee and W. Zhang, Sci. Rep., 2016, 6, 20264 CrossRef CAS PubMed.
- G. Nagaraju, G. S. R. Raju, Y. H. Ko and Y. Jae Su, Nanoscale, 2016, 8, 812–825 RSC.
- W. Du, Z. Zhu, Y. Xu, Z. Zhang, X. Xiong, P. Geng and H. J. Pang, Solid State Electrochem., 2015, 7, 2177–2188 CrossRef.
- Y. Zhang, C. Sun and H. Su, Nanoscale, 2015, 7, 3155–3163 RSC.
- J. Xiao, L. Wan, S. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831–838 CrossRef CAS PubMed.
- L. Mei, T. Yang, C. Xu, M. Zhang, Q. Li and t. Wang, Nano Energy, 2014, 3, 36–45 CrossRef CAS.
- F. Tao, Y. Zhao, G. Zhang and H. Li, Electrochem. Commun., 2007, 9, 1282–1287 CrossRef CAS.
- L. Zhang, H. B. Wu and X. W. Lou, Chem. Commun., 2012, 48, 6912–6914 RSC.
- K. Tao, P. Li, L. Kang, X. Li, Q. Zhou, L. Dong and W. Liang, J. Power Sources, 2015, 293, 23–32 CrossRef CAS.
- W. Du, Z. Wang, Z. Zhu, S. Hu, X. Zhu, Y. Shi, H. Pang and X. Qian, J. Mater. Chem. A, 2014, 2, 9613–9619 CAS.
- D. Aradilla, D. Gaboriau, G. Bidan, P. Gentile, M. Boniface, D. Dubal, P. Gómez-Romero, J. Wimberg, T. J. S. Schubert and S. Sadki, J. Mater. Chem. A, 2015, 3, 13978–13985 CAS.
- C. K. Ranaweera, Z. Wang, E. Alqurashi, P. K. Kahol, P. R. Dvornic, B. K. Gupta, K. Ramasamy, A. D. Mohite, G. Gupta and R. K. Gupta, J. Mater. Chem. A, 2016, 4, 9014–9018 CAS.
- T. Zhu, G. Zhang, T. Hu, Z. He, Y. Lu, G. Wang, H. Guo, J. Luo, C. Lin and Y. Chen, J. Mater. Sci., 2016, 51, 1903–1913 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20871a |
| This journal is © The Royal Society of Chemistry 2016 |