Towards bulk syntheses of nanomaterials: a homeostatically supersaturated synthesis of polymer-like Bi2S3 nanowires with nearly 100% yield and no injection†
Bin Yuanab,
Jordan Aaron Brandta,
Santosh Shawa,
Pratyasha Mohapatraa and
Ludovico Cademartiri*abc
aDepartment of Materials Science & Engineering, Iowa State University of Science and Technology, 2220 Hoover Hall, Ames, IA 50011, USA. E-mail: lcademar@iastate.edu
bDepartment of Chemical & Biological Engineering, Iowa State University of Science and Technology, Sweeney Hall, Ames, IA 50011, USA
cAmes Laboratory, U. S. Department of Energy, Ames, IA 50011, USA
First published on 25th November 2016
Abstract
This paper reports the implementation of a one-pot strategy for the synthesis of polymer-like Bi2S3 nanowires from supersaturated precursors. These conditions result in (i) a homeostatically regulated supersaturation of the growing phase during most of the reaction, (ii) a nearly 100% conversion of the limiting reagent, and (iii) an improved colloidal stability and polydispersity of the product (when compared to the hot-injection product) that allows the identification of three new exciton transitions in the absorption spectrum (one of them, importantly, being a weakly absorbing ground state at 1.64 eV). Three different commercial sources of ligands do not yield significantly different conversion rates. Scalability is further improved by lack of stirring after the initial stage of reaction and a lower reaction temperature (90 °C).
We are interested in using colloidal nanoparticles as bulk materials. We1 and others2–5 believe that colloidal nanoparticles can lead to genuinely “programmable” materials (e.g., near-monodisperse grains, homogeneous grain shapes, controllable grain arrangement, designed grain boundary composition) that are fundamentally inaccessible by traditional material manufacturing methods.
The poor scalability of the syntheses of colloidal nanoparticles – especially when compared to traditional methods for the production of powders and slurries – is one of the key bottlenecks and is somewhat inherent to the most common protocols for the synthesis of nanoparticles due to four main issues: mass transport constraints, effect of impurities, low yields, and the high cost of the reagents.
Avoiding mass transport bottlenecks is often pursued by converting injection strategies into one-pot approaches. These are typically “heat-up” methods6,7 or attractive “slow addition” methods.8 Fascinating microfluidic approaches aim to pursue scalability by parallelizing the synthesis.9 Improving reproducibility in spite of technical reagents is often challenging. Many practitioners in this field have lamented, in private, how commercial sources often change their provider for a certain ligand without warning, often forcing laboratories to reoptimize reaction protocols. Increasing yields is often thought to require a necessary compromise: letting the reactions run to completion typically involves larger particle sizes, and the onset of low supersaturations, that typically cause Ostwald ripening10 and an increased particle polydispersity.11 High quality particles are usually obtained instead by quenching the reaction at the end of the focusing phase, when the supersaturation is still high12 and growth is kinetically driven. An approach to circumvent this conundrum lies in using precursors in very high concentration, well above saturation.13 These routes, that we called “heterogeneous reaction routes” might have been first demonstrated by Joo et al. who used PbCl2 in oleylamine (OLA) as a precursor for PbS nanocrystals.14,15 Later work showed that using slurries of sparingly soluble precursors can yield remarkable product quality and scalability.16 Recent work confirmed the virtues of high concentrations of precursors in obtaining high quality nanoparticles.17 Supersaturating one precursor (especially if in excess) ensures that the concentration of that precursor remains constant during the reaction. This approach does not require a change in the volume of the reaction, it does not produce localized bursts of supersaturation capable of producing secondary nuclei, and it homeostatically matches the delivery of available precursor to the rate of its consumption. While several syntheses have now been reported with supersaturated precursors,16 none of them, to our knowledge, have used a one-pot approach and supersaturated both precursors to create a genuinely constant and homeostatic supersaturation throughout growth (at least until exhaustion of the limiting reagent).
Research interest in Bi2S3 nanomaterials is spurred by potential applications in a wide variety of fields, such as computed tomography imaging,18 solar cells,19 photocatalysis,20 photodetectors,21 and biomolecules detection.22 These applications originate from the special properties of Bi2S3, such as low toxicity, low cost, relatively narrow and direct band gap (∼1.4 eV), high electron density and refractive index (∼4), and good thermoelectric properties. We,1,23 and others,24 are interested in nanowires for their similarities (both in morphology, growth mechanism, and physical properties) with polymer molecules. In the case of ultrathin (diameter < 3–5 nm) colloidal nanowires, it appears that the similarities are not limited to the 1D morphology. For example, the growth kinetics of some of these nanowires (ultrathin Bi2S3 nanowires) was shown to be describable quantitatively with step-growth polymerization models that include addition of monomers to the tips and coupling of fully-formed nanowires end-to-end.23
The potential for application (e.g., in flexible electronics and photonics, biocompatible devices, smart paints25) for combining in a single phase the properties that are traditionally considered unique to polymers (such as viscoelasticity, reptation) with the physical properties of inorganic nanowires is massive, but strongly dependent on the ability of synthesizing these materials on a large scale.
We here report on the synthesis of polymer-like Bi2S3 nanowires using a one-pot approach in fully supersaturated conditions of both precursors (bismuth citrate and elemental sulfur). This approach to nanoparticle synthesis combines for the first time the scalability of one-pot approaches with the high quality, reliability, simplicity, and sustainability of heterogeneous reaction approaches. The result is a reaction that can produce grams of high quality material in a single step, with minimal use of solvent, with nearly 100% yield, and with nearly complete recyclability of the unused reagents.
The starting point for our reaction design was a previously reported hot injection route.26 Reproducing those reaction conditions with the currently available, technical purity OLA (Sigma-Aldrich source) yields, in many cases, relatively short (100–200 nm in length) wires.
The one-pot route was carried out by combining bismuth citrate, elemental sulfur, and OLA in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 molar ratio (compared to the 1
30 molar ratio (compared to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 ratio of the hot injection route) in a 90 °C oil bath as shown in Fig. 1a and ESI.† As both precursors are insoluble in OLA at these concentrations, the reaction is supersaturated in both of the precursors while bismuth citrate is the limiting reagent. The comparison of the nanowires produced by hot injection (OLA from Sigma-Aldrich) and the ones produced by one-pot (OLA from Acros) at different stages of growth is shown in Fig. 1b and c, respectively. Both approaches yielded the expected long, 1.6 nm-thick nanowires. TEM images taken under higher magnification were shown in ESI.† XRD spectra were consistent with those of the previously reported product (cf. ESI†).26 The TEM images show that the nanowires grown in the one-pot route grow significantly longer than those in the hot injection route.
14 ratio of the hot injection route) in a 90 °C oil bath as shown in Fig. 1a and ESI.† As both precursors are insoluble in OLA at these concentrations, the reaction is supersaturated in both of the precursors while bismuth citrate is the limiting reagent. The comparison of the nanowires produced by hot injection (OLA from Sigma-Aldrich) and the ones produced by one-pot (OLA from Acros) at different stages of growth is shown in Fig. 1b and c, respectively. Both approaches yielded the expected long, 1.6 nm-thick nanowires. TEM images taken under higher magnification were shown in ESI.† XRD spectra were consistent with those of the previously reported product (cf. ESI†).26 The TEM images show that the nanowires grown in the one-pot route grow significantly longer than those in the hot injection route.
The quality and colloidal stability of the product is especially good and results in an improved resolution of the fine structure of the absorption spectrum, which can be discerned by second derivative analysis. The high quality of the Bi2S3 nanowires can presumably be attributed to the sustainability of the one-pot reaction (i.e. the reaction proceeded in a steady manner without Ostwald ripening). Fig. 2 shows a representative spectrum of an aliquot from the one-pot route. The second derivative analysis allows for the identification of 6 exciton transitions (labeled in Fig. 2 as E1 to E6 from the ground state up), three of which (E1, E4, and E6) previously unknown. While the features associated with some of the transitions (E1, E3, E4, and E6) are weak, their reproducible observation in more than 10 consecutive samples collected at different times from the reaction mixture in separate reactions strongly suggests them to be real features of the spectrum. E1* indicates the expected bandgap energy of the bulk phase (886 nm, 1.4 eV, E1*).27,28 The constant energy of the transitions (cf. ESI†) during growth demonstrates that the nanowires grow only in length: computational work indicates that quantum confinement effects in Bi2S3 only become significant below 3 nm.27,28
The comparison of the energy levels shown in Fig. 2 and those reported in our previous publication26 indicate that what we originally believed to be the ground state for the exciton is actually E2 and that a lower energy level exists, albeit with what appears to be a much lower oscillator strength. While the energy of this ground state (E1 = 1.64 eV) is lower than that previously reported in 2008 (1.88 eV), it is still higher than the bandgap calculated for Bi2S3 nanowires (1.4–1.5 eV). Based on the elegant computational work by Calzia et al.28 and Aresti et al.,27 this difference could be attributed to the polycrystallinity of the wires.26 The larger-than-expected energy of the first excitonic transition does suggest that the nanowires are well-coordinated and devoid of significant sulfur vacancies, as supported by EXAFS and XANES29 characterization. The frustrating and mystifying lack of radiative recombination in these wires (Bi2S3 is a direct-bandgap semiconductor28) might be due to traps that originate from grain boundaries or amorphous regions within the nanowires. The relatively large energy of E1 suggest that these traps, if they do exist, do not introduce deep intragap states. Nonetheless we encourage caution in expecting quantitative matching between simulation and experimental data in this system. For example, (i) strain is observed in the simulated wires, while no strain was observed in the XRD, EXAFS and XANES spectra;29 (ii) the binding energy of carboxylic acids to the wires is calculated to be larger than that of amines, but our experience indicates that carboxylic acids do not displace amines from the surface, in any concentration.30
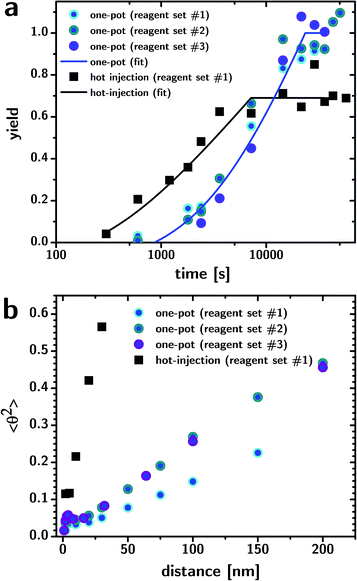
It is important to test the reproducibility and robustness of colloidal nanoparticle synthesis against significant changes in the reagent sources. Fig. 3a shows the yield of the reaction (determined from optical absorbance, using the previously determined extinction coefficient26) during growth for one-pot reactions that use three different sources of OLA and precursors (reagent set #1; OLA, Bi citrate, S8 from Sigma Aldrich; #2; OLA from Acros, Bi citrate from Sigma-Aldrich, S8 from Sigma-Aldrich; #3; OLA from ChemCruz, Bi citrate from Amresco, S8 from Acros, round scatters), compared to the yield observed with hot-injection (reagent set #1, square scatters). While the yield of the hot-injection route appears to cap at ∼60–70%, the yield of the one-pot route reliably reaches nearly 100% (or 6.5 g of product for a ∼300 mL reaction). The limited yield in the injection route might be due to S-containing reaction byproducts stable towards reaction with bismuth and decomposition to H2S. The three one-pot reactions gave very similar conversion kinetics with a longer onset time than the hot-injection.
The lines in Fig. 3a are fits using the “step-growth-like” growth kinetic model described in a previous publication.23 The model consists of two mechanisms: the addition process adds monomers to the tips of the nanowires; the coupling process couples nanowires end to end. This very simple model effectively explains the logarithmic dependency of yield on time, which is displayed by both the hot-injection and one-pot routes. The fits shown in Fig. 3a were obtained by assuming the rate constants of addition and coupling to be the same for both reaction strategies (Tgrowth was the same at 90 °C). The fitted values of the constants (kaddition = 3.5 × 10−5 mol m−2 s−1 and kcoupling = 1.69 × 103 M−1 s−1) are consistent with the values previously reported for reactions conducted at 100 °C (5 ± 4 × 10−5 mol m−2 s−1 and 4 ± 2 × 103 M−1 s−1 respectively).23 The onset time (300 s for hot injection and 1450 s for the one-pot) and the concentration of nanowires at the onset time (1 × 10−7 M for one-pot and 1 × 10−6 M for hot injection versus 1 × 10−6 M for hot injection previously reported23) were allowed to differ between the reactions strategies. The longer onset time and the smaller number of nanowires formed in the one-pot approach is consistent with a slower onset of nucleation due to slowly increasing temperatures. The sudden halt in the conversion, rather than a sigmoidal plateauing, is consistent with a reaction occurring between two supersaturated reagents, of which one is limiting and where the dissolution kinetics of the precursor are not a slow step: the supersaturation remains constant until the limiting precursor is not supersaturated anymore, at which point the available precursor is quickly exhausted. It is worth noting that even though stirring stopped during the conversion of the precursors, the kinetics of the reaction did not show obvious changes, as shown in Fig. 3a.
While the ability of the model to fit the mostly logarithmic kinetic of the conversion is consistent with previous work, the observed lengths of the nanowires are, in this case, shorter than predicted by the model. Termination, fragmentation, and secondary nucleation processes can explain the somewhat shorter wires, but they would introduce substantial deviations in the conversion kinetics. A better understanding of the growth kinetic will require the characterization of these nanowires prepared from different, simpler sulfur precursors at different temperatures.
Beside nanowire length, the persistence length is another important measure of the nanowire morphology and can be determined from TEM images by plotting the average angle squared between adjacent vectors that are created by connecting equally spaced adjacent points (space = l) on the “backbone”, against the space l.31 The slope is the inverse of the persistence length p according to the equation 〈θ2〉 = l/p. The data shows that the nanowires obtained from the one-pot synthesis are fairly reproducible (p = 659 nm, 443 nm, and 421 nm) and much straighter than the hot-injection counterparts (p = 50 nm). The persistence length in the hot-injection sample is fairly consistent with the one reported from a 100 °C reaction (14 nm).23 The discrepancy suggests that the persistence length is a very plastic morphological parameter and subtly sensitive to minute changes in the reaction conditions (e.g., nanowire concentration, temperature of growth) or handling (e.g., stirring). A more systematic investigations of the parameters affecting the persistence length of the wires is underway.
In conclusion, Bi2S3 nanowires were synthesized using a one-pot strategy with almost 100% conversion rate of the limiting reagent. The reaction involves a homeostatically regulated supersaturation of both precursors. Three new exciton transitions in the absorption spectrum of the nanowires were identified. The one-pot reaction approach was robust and did not depend on the commercial sources of ligands (OLA) and precursors.
Acknowledgements
The work was sponsored by Iowa State University through a startup grant to LC.Notes and references
- L. Cademartiri and K. J. M. Bishop, Nat. Mater., 2015, 14, 2–9 CrossRef CAS PubMed.
- D. Frenkel, Nat. Mater., 2015, 14, 9–12 CrossRef CAS PubMed.
- M. V. Kovalenko, L. Manna, A. Cabot, Z. Hens, D. V. Talapin, C. R. Kagan, V. I. Klimov, A. L. Rogach, P. Reiss, D. J. Milliron, P. Guyot-Sionnnest, G. Konstantatos, W. J. Parak, T. Hyeon, B. A. Korgel, C. B. Murray and W. Heiss, ACS Nano, 2015, 9, 1012–1057 CrossRef CAS PubMed.
- R. L. Whetten, J. T. Khoury, M. M. Alvarez, S. Murthy, I. Vezmar, Z. L. Wang, P. W. Stephens, C. L. Cleveland, W. D. Luedtke and U. Landman, Adv. Mater., 1996, 8, 428–434 CrossRef CAS.
- S. C. Glotzer and M. J. Solomon, Nat. Mater., 2007, 6, 557–562 CrossRef PubMed.
- S. G. Kwon, Y. Piao, J. Park, S. Angappane, Y. Jo, N.-M. Hwang, J.-G. Park and T. Hyeon, J. Am. Chem. Soc., 2007, 129, 12571–12584 CrossRef CAS PubMed.
- Y. C. Cao and J. H. Wang, J. Am. Chem. Soc., 2004, 126, 14336–14337 CrossRef CAS PubMed.
- D. Ito, S. Yokoyama, T. Zaikova, K. Masuko and J. E. Hutchison, ACS Nano, 2014, 8, 64–75 CrossRef CAS PubMed.
- S. Marre and K. F. Jensen, Chem. Soc. Rev., 2010, 39, 1183–1202 RSC.
- P. W. Voorhees, J. Stat. Phys., 1985, 38, 231–252 CrossRef.
- D. V. Talapin, A. L. Rogach, M. Haase and H. Weller, J. Phys. Chem. B, 2001, 105, 12278–12285 CrossRef CAS.
- X. G. Peng, J. Wickham and A. P. Alivisatos, J. Am. Chem. Soc., 1998, 120, 5343–5344 CrossRef CAS.
- L. Cademartiri, J. Bertolotti, R. Sapienza, D. S. Wiersma, V. Kitaev and G. A. Ozin, J. Phys. Chem. B, 2006, 110, 671–673 CrossRef CAS PubMed.
- J. Joo, H. B. Na, T. Yu, J. H. Yu, Y. W. Kim, F. Wu, J. Z. Zhang and T. Hyeon, J. Am. Chem. Soc., 2003, 125, 11100–11105 CrossRef CAS PubMed.
- While the paper describes a “clear and homogeneous solution”, we have never been able to obtain it and we suspect the observation might have been misstated.
- L. Cademartiri and G. A. Ozin, Philos. Trans. R. Soc., A, 2010, 368, 4229–4248 CrossRef CAS PubMed.
- C. B. Williamson, D. R. Nevers, T. Hanrath and R. D. Robinson, J. Am. Chem. Soc., 2015, 137, 15843–15851 CrossRef CAS PubMed.
- O. Rabin, J. M. Perez, J. Grimm, G. Wojtkiewicz and R. Weissleder, Nat. Mater., 2006, 5, 118–122 CrossRef CAS PubMed.
- A. K. Rath, M. Bernechea, L. Martinez and G. Konstantatos, Adv. Mater., 2011, 23, 3712–3717 CrossRef CAS PubMed.
- Z. Zhang, W. Wang, L. Wang and S. Sun, ACS Appl. Mater. Interfaces, 2012, 4, 593–597 CAS.
- G. Konstantatos, L. Levina, J. Tang and E. H. Sargent, Nano Lett., 2008, 8, 4002–4006 CrossRef CAS PubMed.
- L. Cademartiri, F. Scotognella, P. G. O'Brien, B. V. Lotsch, J. Thomson, S. Petrov, N. P. Kherani and G. A. Ozin, Nano Lett., 2009, 9, 1482–1486 CrossRef CAS PubMed.
- L. Cademartiri, G. Guerin, K. J. M. Bishop, M. A. Winnik and G. A. Ozin, J. Am. Chem. Soc., 2012, 134, 9327–9334 CrossRef CAS PubMed.
- S. Hu and X. Wang, Chem. Soc. Rev., 2013, 42, 5577–5594 RSC.
- B. Yuan and L. Cademartiri, J. Mater. Sci. Technol., 2015, 31, 607–615 Search PubMed.
- L. Cademartiri, R. Malakooti, P. G. O'Brien, A. Migliori, S. Petrov, N. P. Kherani and G. A. Ozin, Angew. Chem., Int. Ed., 2008, 47, 3814–3817 CrossRef CAS PubMed.
- M. Aresti, M. Saba, R. Piras, D. Marongiu, G. Mula, F. Quochi, A. Mura, C. Cannas, M. Mureddu, A. Ardu, G. Ennas, V. Calzia, A. Mattoni, A. Musinu and G. Bongiovanni, Adv. Funct. Mater., 2014, 24, 3341–3350 CrossRef CAS.
- V. Calzia, G. Malloci, G. Bongiovanni and A. Mattoni, J. Phys. Chem. C, 2013, 117, 21923–21929 CAS.
- J. W. Thomson, L. Cademartiri, M. MacDonald, S. Petrov, G. Calestani, P. Zhang and G. A. Ozin, J. Am. Chem. Soc., 2010, 132, 9058–9068 CrossRef CAS PubMed.
- L. Cademartiri, F. Scotognella, P. G. O'Brien, B. V. Lotsch, J. Thomson, S. Petrov, N. P. Kherani and G. A. Ozin, Nano Lett., 2009, 9, 1482–1486 CrossRef CAS PubMed.
- C. Frontali, E. Dore, A. Ferrauto, E. Gratton, A. Bettini, M. R. Pozzan and E. Valdevit, Biopolymers, 1979, 18, 1353–1373 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods. Energy levels and normalized confinement energies as a function of reaction time. XRD spectra of hot-injection and one-pot products. Video of the product handling. See DOI: 10.1039/c6ra20772c |
| This journal is © The Royal Society of Chemistry 2016 |