Tandem additions of 3,4-dihydroisoquinolines to γ-hydroxy-α,β-unsaturated ketones: a green and new access to oxazolo[2,3-a]tetrahydroisoquinolines†
Sheng-Han Huang,
Yu-Wei Shih,
Wen-Tse Huang,
Deng-Hong Li and
Te-Fang Yang*
Department of Applied Chemistry, National Chi Nan University, 1, University Road, Puli, 545, Nantou, Taiwan. E-mail: tfyang@ncnu.edu.tw
First published on 21st September 2016
Abstract
Addition reaction of 3,4-dihydroisoquinolines with γ-hydroxy-α,β-unsaturated ketones furnished a variety of diastereomerically pure oxazolo[2,3-a]tetrahydro-isoquinolines, which could be purified by either recrystallization or solvent evaporation. The reaction proceeded smoothly under “green” conditions without an additive and catalyst, giving the target molecules in good to excellent yields.
It is well known that oxazolidine derivatives are highly important heterocycles with several uses for organic synthesis. For example, 1,3-oxazolidine derivatives have been generally used as ligand(s) for the metal-catalyzed asymmetric reactions.1 On the other hand, some of the oxazolidines are unique intermediates for the synthesis of stereo-specific amino alcohols.2 Furthermore, the oxazolidine moiety is an essential part of the molecular structure of natural products, such as quinocarcin,3 naphthyridinomycin4 and tetrazomine,5 which have been found to be potential antitumor agents.6 Hence, to search for efficient methods for the syntheses of oxazolidines,1,2,7–9 and their analogs, such as oxazolo-pyrrolidine,10 fused oxazolidines11 and oxazoloisoquinolines12 is of great importance to organic chemists.
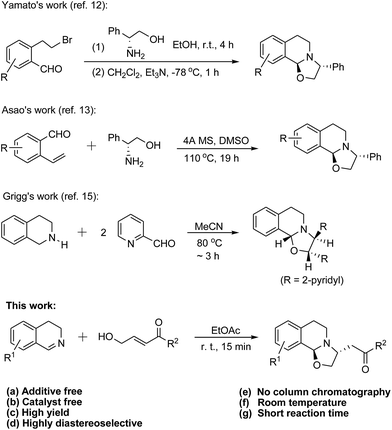
Especially, oxazolo[2,3-a]tetrahydroisoquinolines have been important intermediates for the synthesis of isoquinoline alkaloids. By starting from isochroman, as shown in Scheme 1, Yamato et al. accomplished the diastereoselective synthesis of oxazolo[2,3-a]tetrahydroisoquinoline,12 which was obtained in three steps then converted to chiral 1-substituted tetrahydroisoquinolines. In addition, 3-phenyl oxazolo[2,3-a]tetrahydroisoquinoline was afforded by the condensation of ortho-vinylbenzaldehyde with (R)-phenylglycinol in hot 1,4-dioxane or DMSO.13 Furthermore, a similar synthetic methodology was applied to the preparation of oxazolo[2,3-a]tetrahydroisoquinolone, a tricyclic lactam.14 On the other hand, Grigg and co-workers studied the reaction of two equivalents of pyridine-2-carbaldehyde with 1,2,3,4-tetrahydroisoquinoline in warm acetonitrile, and found that dipyridyl oxazolo[2,3-a]tetrahydroisoquinoline was furnished.15 The mechanism of this reaction involved a formation of an intermediate called azomethine ylide. Recently, Houk, Seidel and co-workers described that the reaction of salicylaldehyde with tetrahydrohydroisoquiline could provide diphenolic oxazolo[2,3-a]tetrahydroisoquinoline, which was a [3 + 2] adduct.16 In addition, it is particularly noteworthy that Matsubara and co-workers carried out the asymmetric cycloaddition reaction of N-tosyl imines with γ-hydroxy-α,β-unsaturated ketones in the presence of a bifunctional catalyst derived from cinchonidine.8,17 All the interesting contents of reports mentioned above prompted us to search for a sustainable and efficient methodology for the synthesis of oxazolo[2,3-a]tetrahydroisoquinolines. Thus, we report herein the results of the addition of 3,4-dihydroisoquinolines with γ-hydroxy-α,β-unsaturated ketones (Scheme 1). To the best of our knowledge, this is the first example of the synthesis of oxazolo[2,3-a]tetrahydroisoquinoline derivatives via a reaction carried out under environmentally friendly conditions.18
For the condition screening, 3,4-dihydroisoquinoline (1a) and (E)-4-hydroxy-1-phenylbut-2-en-1-one (2a) were utilized as the reactants of the model reaction (Table 1). It is noteworthy that Jacobsen and co-workers reported that 3,4-dihydroisoquinoline could be activated with acetic acid to an iminium ion, which then reacted with a dienanine in toluene to afford benzoquinolizidine.19 At beginning of our investigation, hence, toluene was selected as the solvent for the addition of 3,4-dihydroisoquinoline (1a) with (E)-4-hydroxy-1-phenylbut-2-en-1-one (2a) in the presence of acetic acid (15 mol%), which might function as the additive or catalyst (entry 1). Then, it was found that product 3a could be also obtained in an acceptable yield in the absence of acetic acid (entries 2, 3). The yield of 3a remained almost unchanged either with or without acetic acid even if the reaction time was decreased to 15 min (entries 4, 5). Compared with toluene, both ether and EtOAc are better solvents for the reaction (entries 6–8).
| Entry | Solventa | Additiveb | Time | Yieldc (%) |
|---|---|---|---|---|
| a Conditions: 3,4-dihydroisoquionline (0.76 mmol) and (E)-4-hydroxy-1-phenylbut-2-en-1-one (0.76 mmol) were mixed in solvent (1 mL).b AcOH (15 mol%) was added.c Isolated yield. | ||||
| 1 | Toluene | AcOHb | 1 h | 76 |
| 2 | Toluene | None | 1 h | 73 |
| 3 | Toluene | None | 3 h | 76 |
| 4 | Toluene | AcOHb | 15 min | 75 |
| 5 | Toluene | None | 15 min | 73 |
| 6 | EtOAc | AcOHb | 15 min | 93 |
| 7 | EtOAc | None | 15 min | 93 |
| 8 | Ether | None | 15 min | 83 |
| 9 | H2O | None | 15 min | 75 |
| 10 | MeOH | AcOHb | 15 min | 71 |
| 11 | MeOH | None | 15 min | 73 |
| 12 | EtOH | None | 15 min | 63 |
| 13 | n-BuOH | None | 15 min | 73 |
| 14 | DMF | None | 15 min | 55 |
Especially, the reaction furnished 3a in an excellent yield in EtOAc at room temperature in the absence of additive and catalyst (entry 7). Polar solvents, such as methanol (entries 10, 11) and ethanol (entry 12) could not help improve the efficiency of the addition. Butanol (entry 13) and DMF (entry 14) turned out to be relatively inappropriate solvents for the reaction.
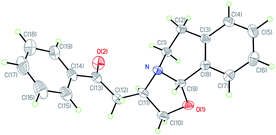
The NMR technique and HRMS experiments were utilized for the characterization of the isolated product. Furthermore, the relative stereo-structure of a single crystal of compound 3a was confirmed with X-ray crystallography (Fig. 1). It revealed that the orientations of the two protons on chiral centres C-9 and C-11 were anti to each other.15a
For the investigation of the scope of substrates, at first, the reactions of various 3,4-dihydroisoquinoline20 derivatives with (E)-4-hydroxy-1-phenylbut-2-en-1-one (2a) were performed under the optimized conditions shown in Table 1 (entry 7). As presented in Table 2, all the reactions were finished at room temperature in 15 min, providing the corresponding products in good to excellent yields. It was found that the reaction tolerated both activating groups (3a–3h) and deactivating groups (3i–3l), which were on the benzo-moiety of the parent dihydroisoquinoline, regardless of their substitution positions. As expected, 6,7-dimethoxy-3,4-dihydroisoquinoline also underwent the reaction to smoothly afford product 3d (95% yield). Notably, it seemed that the phenyl group at C-7 atom of the molecule (3h) did not cause any steric hindrance to the addition. The diastereomeric ratio (dr) for each product was determined based on its proton NMR spectrum. It is noteworthy that the addition reaction was highly diastereoselective (3f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dr > 25
dr > 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
| a dr = diastereomeric ratio determined with 1H-NMR spectrum. |
|---|
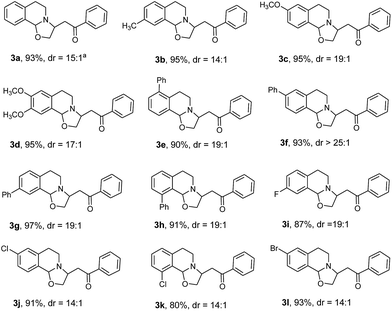 |
With the good results obtained from the above experiments in hand, we subsequently made a move to the exploration of the scope of γ-hydroxy-α,β-unsaturated ketones.21 As presented in Table 3, the addition of various 3,4-dihydroisoquinoline derivatives to the p-methoxyphenyl ketone gave the corresponding oxazolo[2,3-a]tetrahydroisoquinolines (4a–4d) in high to excellent yields. Moreover, products 4e–4g and 4h–4k were obtained easily from the reaction of the p-chlorophenyl ketone and that of the p-nitrophenyl ketone, respectively. Thus, it should be noted that the addition reaction tolerated both activating and deactivating groups on the para-position of phenyl moiety of the applicable γ-hydroxy-α,β-unsaturated ketones 2. Gratifyingly, the reaction of 2-naphthyl ketone 2 with dihydroisoquinolines 1 also proceeded very well and smoothly afforded target molecules 4l–4p (90–93% yield). On the other hand, the reactivity of ketones 2 which possess an alkyl group (R2) instead of an aryl was examined. It was observed that methyl ketone 2 reacted with the dihydroisoquinolines efficiently, providing expected products 4q–4t in high yields. Furthermore, compounds 4u–4x were also easily furnished from the consecutive additions, indicating that the reaction tolerated steric-demanding t-butyl unit on the ketone molecule. The relative stereochemistry of some products was confirmed with NOESY experiments, which provided the information that the NOE between H-9 and H-11 was not observed.
| a dr = diastereomeric ratio, which was determined with 1H-NMR spectrum. |
|---|
 |
As shown in Scheme 2, the addition reaction could be efficiently scaled up under the standard conditions. Reaction of 6-chloro-3,4-dihydroisoquinoline (12.7 mmol) with 2a (12.7 mmol) took 15 min at room temperature, and provided oxazolo[2,3-a]tetrahydroisoquinoline 3j in 85% yield (3.55 g). One might expertize on the potential application of this methodology to the preparation of key intermediates for some natural products or biological active compounds.
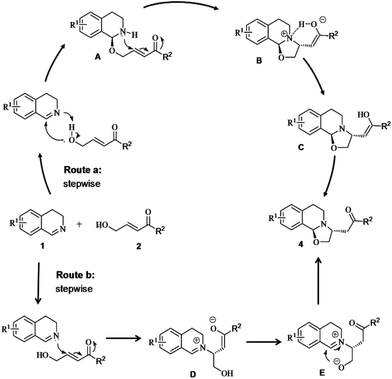
Two plausible mechanisms for the title reaction are illustrated in Fig. 2. At first, it was speculated that the title reaction consisted of two consecutive additions. For route a, regular nucleophilic addition of 1 with 2 furnished structure A,8 which immediately underwent intramolecular aza-Michael addition to give species B. Then, an intramolecular proton-transfer from the ammonium moiety to enolate proceeded in B to afford enol C. Product 4 was then obtained after a tautomerism occurred to enol C. At beginning of the formation of structure A, the oxygen atom of the hydroxyl group in 2 attacked the carbon atom of imino group in 1 from one side of the conjugate system. Consequently, the nitrogen atom on the secondary amino group in A attacked the β-carbon of the unsaturated ketone moiety predominantly from the opposite side during the formation of B. Thus, the reaction turned out to be highly diastereoselective. For route b, intermolecular aza-Michael addition of 1 to 2 provided zwitterion D, which immediately underwent tautomerism to furnish zwitterion E. Then, cyclization of E smoothly afforded product 4. The rational of the stereochemistry involved in route b could be similar to that involved in route a.
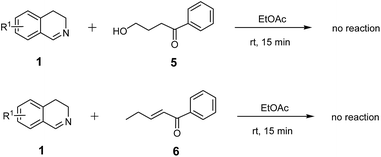
In order to further figure out the exact mechanism for the title reaction, two control experiments were performed under the standard conditions, as shown in Scheme 3. However, both mixture of 1 with 4-hydroxy-1-phenylbut-an-1-one (5)22 and mixture of 1 with (E)-1-phenylpent-2-en-1-one (6)23 revealed no reaction at all. Thus, it is presumed that the attack by the oxygen atom in 2 to the carbon atom of imino group in 1 and the attack by the nitrogen atom of imino group in 1 to the β-carbon of the unsaturated ketone moiety in 2 occurred simultaneously and followed two opposite directions. This situation could be the rational of the stereochemistry involved in the concerted mechanism (route c, Fig. 3). Therefore, route c cannot be excluded while route a or route b (Fig. 2) exhibits the origin of diastereoselectivity for the reaction.
In conclusion, we developed an efficient method for the diastereoselective synthesis of oxazolo[2,3-a]tetrahydro-isoquinolines. The addition reaction of 3,4-dihydro-isoquinolines with γ-hydroxy-α,β-unsaturated ketones could complete under “green” conditions without additive and catalyst, producing the target molecules in good to excellent yields. The applications of this [3 + 2] cycloaddition reaction to the syntheses of analogues of oxazolo[2,3-a]tetrahydro-isoquinolines and other heterocyclic compounds are under investigation.
Experimental section
General procedure for the reaction of 3,4-dihydroisoquinoline derivatives with (E)-4-hydroxy-1-phenyl-but-2-en-1-one (2a) or its analog
To a solution of 3,4-dihydroiso-quinolone or its derivative (1, 0.76 mmol) in EtOAc (1 mL) was added 2a (0.12 g, 0.76 mmol). The reaction mixture was stirred at room temperature for 15 min and, then, concentrated under reduced pressure. After the residue was recrystallized (EtOAc/hexanes), the corresponding product was obtained. The anti-isomer given by each experiment was characterized by 1H-NMR, 13C-NMR, HRMS, etc. Some of the anti-isomers were further characterized by NOESY experiments. The results of these experiments were shown in the ESI.† Products 3a and 3l were further characterized by X-ray crystallography. The other diastereoisomer could not be separated by crystallization.General procedure for the reaction of 3,4-dihydroisoquinoline derivatives (1) with (E)-4-hydroxy-1-alkyl-but-2-en-1-one
To a solution of 3,4-dihydroisoquinolone or its derivative (1, 0.76 mmol) in EtOAc (1 mL) was added 2 (0.12 g, 0.76 mmol). The reaction mixture was stirred at room temperature for 15 min and, then, concentrated under reduced pressure. The residue was the pure target compound for characterization. All the products obtained from this procedure were liquids, and were not available for crystallization. The anti-isomer given by each experiment was characterized by 1H-NMR, 13C-NMR, HRMS, etc. Some of the anti-isomers were further characterized by NOESY experiments. The results of these experiments were shown in the ESI.†Acknowledgements
This work was supported by Ministry of Science and Technology, Taiwan (Grant MOST 103-2113-M-260-004), which is gratefully acknowledged. The authors also thank the Instrumental Centre at National Taiwan University and the Regional Instruments Centre at National Chung-Hsing University for high resolution mass spectra and X-ray crystallography.Notes and references
- C. Wolf and H. Xu, Chem. Commun., 2011, 47, 3339 RSC ; and the references therein.
- (a) K. S. Williamson and T. P. Yoon, J. Am. Chem. Soc., 2012, 134, 12370 CrossRef CAS PubMed; (b) D. J. Michaelis, K. S. Williamson and T. P. Yoon, Tetrahedron, 2009, 65, 5118 CrossRef CAS PubMed.
- (a) R. M. Williams, T. Glinka, M. E. Flanagan, R. Gallegos, H. Coffman and D. Pei, J. Am. Chem. Soc., 1992, 114, 733 CrossRef CAS; (b) F. Tomita, K. Takahashi and K. Shimizu, J. Antibiot., 1983, 36, 463 CrossRef CAS PubMed; (c) M. Hirayama and K. Shirahata, J. Chem. Soc., Perkin Trans. 2, 1983, 1705 RSC; (d) F. Tomita, K. Takahashi and T. Tamaoki, J. Antibiot., 1984, 37, 1268 CrossRef CAS PubMed.
- D. Kluepfel, H. A. Baker, G. Piattoni, S. N. Sehgal, A. Sidorowicz, K. Singh and C. Vezina, J. Antibiot., 1975, 28, 497 CrossRef CAS PubMed.
- K. Suzuki, T. Sato, M. Morioka, K. Nagai, K. Abe, H. Yamaguchi, T. Saito, Y. Ohmi and K. Susaki, J. Antibiot., 1991, 44, 479 CrossRef CAS PubMed.
- J. D. Scott and R. M. Williams, Chem. Rev., 2002, 102, 1669 CrossRef CAS PubMed ; and the references therein.
- (a) S. K. Nimmagadda, Z. Zhang and J. C. Antilla, Org. Lett., 2014, 16, 4098 CrossRef CAS PubMed; (b) Z. Liu, X. Feng and H. Du, Org. Lett., 2012, 14, 3154 CrossRef CAS PubMed; (c) M. B. Shaghafi, R. E. Grote and E. R. Jarvo, Org. Lett., 2011, 13, 5188 CrossRef CAS PubMed.
- Y. Fukata, K. Asano and S. Matsubara, Chem. Lett., 2013, 42, 355 CrossRef CAS.
- (a) S. Murru, C. S. Lott, B. McGough, D. M. Bernard and R. S. Srivastava, Org. Biomol. Chem., 2016, 14, 3681 RSC; (b) K. M. Allan and B. M. Stoltz, J. Am. Chem. Soc., 2008, 130, 17270 CrossRef CAS PubMed; (c) J.-G. Shim and Y. Yamamoto, Heterocycles, 2000, 52, 885 CrossRef CAS; (d) J.-G. Shim and Y. Yamamoto, Tetrahedron Lett., 1999, 40, 1053 CrossRef CAS; (e) A. F. Ward and J. P. Wolfe, Org. Lett., 2011, 13, 4728 CrossRef CAS PubMed; (f) S. Gandhi, A. Bisai, B. A. Prasa and V. K. Singh, J. Org. Chem., 2007, 72, 2133 CrossRef CAS PubMed; (g) D. J. Michaelis, M. A. Ischay and T. P. Yoon, J. Am. Chem. Soc., 2008, 130, 6610 CrossRef CAS PubMed.
- A. R. Katritzky, X.-L. Cui, B. Yang and P. J. Steel, J. Org. Chem., 1999, 64, 1979 CrossRef CAS PubMed.
- Y. Ukaji, K. Yamamoto, M. Fukui and T. Fujisawa, Tetrahedron Lett., 1991, 32, 2919 CrossRef CAS.
- (a) M. Yamato, K. Hashigaki, S. Ishikawa and N. Qais, Tetrahedron Lett., 1988, 29, 6949 CrossRef CAS; (b) M. Yamato, K. Hashigaki, N. Qais and S. Ishikawa, Tetrahedron, 1990, 46, 5909 CrossRef CAS; (c) K. Hashigaki, K. Kan, N. Qais, Y. Takeuchi and M. Yamato, Chem. Pharm. Bull., 1991, 39, 1126 CrossRef CAS.
- K. Umetsu and N. Asao, Tetrahedron Lett., 2008, 49, 2722 CrossRef CAS.
- M. Amat, V. Elias, N. Llor, F. Subrizi, E. Molins and J. Bosch, Eur. J. Org. Chem., 2010, 4017 CrossRef CAS.
- (a) H. Ardill, R. Grigg, V. Sridharan, S. Surendrakumar, S. Thianpatanagul and S. Kanajun, J. Chem. Soc., Chem. Commun., 1986, 602 RSC; (b) M. F. Aly, H. Ardill, R. Grigg, S. Leong-Ling, S. Rajviroongit and S. Surendrakumar, Tetrahedron Lett., 1987, 48, 6077 CrossRef; (c) H. Ardill, X. L. R. Fontaine, R. Grigg, D. Henderson, J. Montgomery, V. Sridharan and S. Surendrakumar, Tetrahedron, 1990, 46, 6449 CrossRef CAS; (d) F. Orsini, F. Pelizzoni, M. Forte, R. Destro and P. Gariboldi, Tetrahedron, 1988, 44, 519 CrossRef CAS.
- M. T. Richers, M. Breugst, A. Y. Platonova, A. Ullrich, A. Dieckmann, K. N. Houk and D. Seidel, J. Am. Chem. Soc., 2014, 136, 6123 CrossRef CAS PubMed.
- (a) K. Asano and S. Matsubara, J. Am. Chem. Soc., 2011, 133, 16711 CrossRef CAS PubMed; (b) K. Asano and S. Matsubara, Org. Lett., 2012, 14, 1620 CrossRef CAS PubMed; (c) T. Okamura, K. Asano and S. Matsubara, Chem. Commun., 2012, 48, 5076 RSC; (d) Y. Fukata, K. Asano and S. Matsubara, J. Am. Chem. Soc., 2013, 135, 12160 CrossRef CAS PubMed.
- S. Elangovan, J.-B. Sortais, M. Beller and C. Darcel, Angew. Chem., Int. Ed., 2015, 54, 14483 CrossRef CAS PubMed.
- M. P. Lalonde, M. A. McGowan, N. S. Rajapaksa and E. N. Jacobsen, J. Am. Chem. Soc., 2013, 135, 1891 CrossRef CAS PubMed.
- The 3,4-dihydroisoquinoline derivatives are commercial available. They could be also prepared using the procedure reported in literature. For the preparation of 3,4-dihydroisoquinoline derivatives, please see: (a) T. M. Bohme, C. E. Augelli-Szafran, H. Hallak, T. Pugsley, K. Serpa and R. D. Schwarz, J. Med. Chem., 2002, 45, 3094 CrossRef PubMed; (b) R. D. Larsen, R. A. Reamer, E. G. Corley, P. Davis, E. J. J. Grabowski, P. J. Reider and I. Shinkai, J. Org. Chem., 1991, 56, 6034 CrossRef CAS; (c) Z. Gao, L. Davis, Y. Chiang, R. Munson and J. A. Hendrix, Tetrahedron Lett., 2012, 53, 4429 CrossRef CAS; (d) J. C. A. Flanagan, L. M. Dornan, M. G. McLaughlin, N. G. McCreanor, M. J. Cook and M. J. Muldoon, Green Chem., 2012, 14, 1281 RSC.
- For the preparation of various γ-hydroxy-α,β-unsaturated ketones, please see: (a) F. N. Palmer and D. K. Taylor, J. Chem. Soc., Perkin Trans. 1, 2000, 1323 RSC; (b) T. D. Avery, D. K. Taylor and E. R. T. Tiekink, J. Org. Chem., 2000, 65, 5531 CrossRef CAS PubMed; (c) B. W. Greatrex, M. C. Kimber, D. K. Taylor and E. R. T. Tiekink, J. Org. Chem., 2003, 68, 4239 CrossRef CAS PubMed; (d) D. R. Li, A. Murugan and J. R. Falck, J. Am. Chem. Soc., 2008, 130, 46 CrossRef CAS PubMed; (e) H. Kato, JP 2008214276, 2008; (f) M. Sada, S. Ueno, K. Asano, K. Nomura and S. Matsubara, Synlett, 2009, 724 CAS.
- S. K. Murphy and V. M. Dong, J. Am. Chem. Soc., 2013, 135, 5553 CrossRef CAS PubMed.
- For the preparation of 6, please see: K. Kumar, S. S. More, S. Goyal, M. Gangar, G. L. Khatik, R. K. Rawal and V. A. Nair, Tetrahedron Lett., 2016, 57, 2315 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization, NMR spectra, single-crystal XRD data. CCDC 1483927–1483929. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra20708a |
| This journal is © The Royal Society of Chemistry 2016 |