Determination of triazine herbicides from honey samples based on hydrophilic molecularly imprinted resins followed by high performance liquid chromatography-tandem mass spectrometry
Abstract
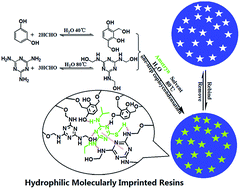
A facile, novel and efficient approach to extract six triazine herbicides from honey samples based on hydrophilic molecularly imprinted resins (MIRs) was described. The MIRs were prepared in a one-pot polycondensation with resorcinol and melamine as the double functional monomers, formaldehyde as a crosslinking agent, and ametryn as the template. The obtained MIRs have a uniform spherical morphology and excellent dispersibility in water due to them having a large number of hydrophilic groups. The MIRs were successfully used as a solid-phase extraction material to rapidly extract and clean up to six triazines in honey samples followed by high performance liquid chromatography-tandem mass spectrometry detection. Under optimal conditions, the method showed higher recoveries and a shorter extraction time. The detection limits of the six triazines are in the range of 0.02–0.15 ng g−1. The recoveries of the six triazines are in the range of 83 ± 4% to 97 ± 4% at the spiked level of 5 ng g−1. The proposed method is simple and quick and has the potential to detect triazine herbicides in other aqueous samples.

 Please wait while we load your content...
Please wait while we load your content...