DOI:
10.1039/C6RA20692A
(Paper)
RSC Adv., 2016,
6, 99297-99305
Characterization of CuSbSe2 crystallites synthesized using a hot injection method
Received
17th August 2016
, Accepted 30th September 2016
First published on 3rd October 2016
Abstract
In this study, a novel and facile hot injection method for the synthesis of single phase CuSbSe2 crystallites was developed by using low toxic triethylene glycol (TEG) as both the solvent and reducing agent and triethylenetetramine (TETA) as co-reducing agent. The effects of the amounts of TETA addition and reaction temperatures on the phase development were investigated. The crystalline structures, morphologies, chemical compositions and optical characterization of the synthesized products were investigated using XRD, TEM, EDS, XPS, and UV-Vis-NIR. A single phase CuSbSe2 crystallites can be obtained by using triethylene glycol as the solvent and reducing agent and triethylenetetramine as co-reducing agent. TETA addition plays a key role in determining the final phase. The presence of the intermediate phase, Cu3SbSe4 phase could be due to the existence of Cu2+, resulting from the insufficient reducibility in the solution. A sufficient amount of TETA can facilitate the reduction of Cu2+ into Cu+, leading to the preformed Cu3SbSe4 phase dissolved and reacted with Sb2Se3 to form CuSbSe2. The obtained CuSbSe2 phase had a direct band gap with the band gap value of 1.06 eV.
1. Introduction
The application of I–III–VI chalcopyrites as thin-film solar cells was developed to reduce cost and improve conversion efficiency. CuInxGa1−xSe2 (CIGS) is one of the most important semiconductor materials because it has many remarkable advantages such as high absorption coefficients, direct band gap semiconductor, good photostability and high conversion efficiency. Hence, it has been used in thin-film photovoltaic cells.1–3 The conversion efficiency for CIGS-based solar cell laboratory devices has reached 22.3% and the module efficiency for large-scale industrial products was also above 14.5%.4–6 Despite the prospect of CIGS thin film technology, the scarcity of elements (In in CIGS) may limit its potential for terawatt scale application.7 Recently, In-free Cu chalcogenides, such as CuSb(S, Se)2 has been presented as an alternative earth-abundant absorber material for CIGS thin film solar cell due to many noteworthy benefits such as low cost, nontoxicity, direct band gap semiconductor with 1.05–1.45 eV and strong optical absorption with absorption coefficients of more than 104 cm−1.8,9
Many solution processes for preparing thin films have been developed, including electrodeposition,10 spray pyrolysis deposition11 and liquid phase deposition.12 These non-vacuum processes can make the deposition process simpler. Therefore, colloidal routes based on spin-coating or printing to fabricate thin film solar cells have attracted much attention due to their potentials to reduce processing equipment costs, obtain the films with well controlled stoichiometry, and achieve high materials utilization.13 Therefore, the development of low cost, large scale and new synthetic method to prepare high quality semiconductor crystallites for ink applications becomes important. However, the systematic study on the synthesis, chemical and optical properties of CuSbSe2 crystallites has rarely been reported.8,9 By far the thermal decomposition method (rapid hot-injection and heating-up methods) has been the most successful and widely used process in preparing metal chalcogenide nanoparticle.14–16 Oleylamine (OLA) was the most widely used solvent for the synthesis of chalcogenide compounds using hot injection method due to its high boiling point and strong chelation and its ability to dissolve metal salt and elemental selenium to form metal–OLA and Se–OLA complexes.17–19 The preparation of a single CuSbS2 phase using hot-injection has been well studied. However, a single CuSbSe2 phase prepared using hot-injection method has never been reported yet to our best knowledge. Liu et al.20 reported that Cu3SbSe3 nanocrystals can be synthesized using OLA as the solvent by a hot-injection method at 190 °C. A single CuSbSe2 phase can only be synthesized at a temperature higher than that of Cu3SbSe3.21 However, as the reaction temperature was raised to above 240 °C, metal antimony impurity was obtained due to its strong reductive ability, which led to Sb3+ tended to transform into metal Sb.22 Moreover, oleylamine was observed to be adsorbed onto the nanocrystallite surface. During sintering the decomposition of oleylamine with its' long chain alkyl group often leads to cracks, discontinuities in the nanocrystallite film and may leave behind unwanted carbonaceous impurities which might have detrimental consequences on the absorber layer performance.23,24 Polyols are compounds with multiple hydroxyl functional groups. At high temperatures, these hydroxyl groups can dedicate multiple hydrogen atoms and transform into an aldehyde groups. The liberated hydrogen atoms could offer electrons and become H+ ions. The elemental selenium and cupric ions are reduced to active selenium ions (Se2−) and cuprous ions (Cu+) by capturing these electrons.25 Moreover, polyols, such as triethylene glycol (TEG), are often used as the common solvents to prepare chalcogenide compounds, such as CdSe,26 CuInSe2 (ref. 27) and Cu(In, Ga)Se2 (ref. 28) because of its special properties, such as low toxicity, high boiling point, mild reducibility and good dissolvability to many metal salts.29 As triethylene glycol is used as reaction solvent, it may act as both solvent and reducing agent, which may be conducive to CuSbSe2 synthesis requiring mild reducing conditions. Triethylenetetramine (TETA) containing two primary and two secondary amines can act as a reducing agent due to the presence of –NH2 groups,30,31 which is same as the role of oleylamine. Chang et al.32 reported that TETA can be used as reducing agent to enhance the formation of CuInSe2 compounds in the colloidal solution. In this study, a novel and facile hot injection method for the synthesis of single phase CuSbSe2 crystallites was developed by using low toxic triethylene glycol (TEG) as both the solvent and reducing agent and triethylenetetramine (TETA) as co-reducing agent. The effects of the amounts of TETA addition and reaction temperatures on the phase development were investigated. The crystalline structures, morphologies, chemical compositions and optical characterization of the synthesized products were investigated using X-ray diffractometer (XRD), transmission electron microscopy (TEM), electron dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), UV-Vis-NIR absorbance spectroscopy.
2. Experimental procedure
2.1 CuSbSe2 powders synthesized using the hot-injection process
One half mmol Cu(CH3COO)2 (98%, Alfa Aesar) and Sb(CH3COO)3 (99.99%, Aldrich) were mixed with 40 ml triethylene glycol (TEG) (99%, Alfa Aesar) and heated to 150 °C for 1 h until the cationic precursors were completely dissolved. One mmol Se (99.99%, Aldrich) was mixed in 80 ml TEG in a 250 ml three-necked flat-bottom flask connected to a reflux condenser and heated to 250 °C under N2 atmosphere (flow rate = 100 ml per minute) until the anionic precursor was completely dissolved. The cationic precursor solution was then injected instantly into the anionic precursor solution with 0, 100, 200 and 400 μl triethylenetetramine (TETA) (97%, Fluka) added as the reducing agent at 250 °C for various periods. After the reaction, the solution temperature was cooled to 60 °C and then ethanol was added to terminate the reaction. This solution was then centrifuged at 6000 rpm for 10 min and washed with ethanol (99.5%, Nihon Shiyaku). Finally, the precipitates were dried at 80 °C.
2.2 Materials' characterization
The crystalline phases were characterized using an X-ray diffractometer (XRD) (Dandong Fangyuan, DX-2700, Sandong, China) with Cu Kα radiation. The scan range was from 15° to 55°, with a scan step of 0.04° and a scan rate of 2.5° per min. Rietveld refinement was carried out using GSAS. The scan range of XRD was from 15° to 80°, with a scan step of 0.02° and a scan rate of 0.25° per min for Rietveld refinement. A scanning electron microscopy (SEM) (Hitachi SU-1510, Tokyo, Japan) was used to observe the morphology and crystallite size. The morphologies and compositions of the products were performed using field emission transmission electron microscopy (FE-TEM, JEOL JEM-2100F) and an energy-dispersive spectrometer (EDS). The X-ray photoelectron spectra (XPS) of the products were collected with a VGS, thermo K-Alpha instrument, with Al Kα X-ray as the excitation source. Room temperature UV-visible (UV-Vis) absorbance spectra were collected using a Jasco V-670 UV-Vis spectrophotometer.
3. Results and discussion
3.1 Influence of TETA addition
Fig. 1 shows the XRD patterns of the products synthesized using hot injection method with different amounts of TETA after reaction at 250 °C for 1 h. For the sample synthesized without addition of TETA, Cu3SbSe4 and Sb2Se3 were the main phases and no CuSbSe2 phase was observed. As 100 μl TETA was added into the solution, CuSbSe2 phase became the main phase accompanied with Cu3SbSe4 and Sb2Se3. As the content of TETA was increased to 200 μl, all the peaks of both samples match well with standard peaks of orthorhombic CuSbSe2 (JCPDS 01-078-8410). Further increasing the content of TETA to 400 μl, a small amount of metal Sb phase appeared in addition to CuSbSe2 phase. These results indicate that a proper amount of TETA addition is required for the pure CuSbSe2 single phase formation. Rietveld refinement was carried out and the result of the sample synthesized with 200 μl TETA is shown in Fig. 1(b). All peaks can be indexed to CuSbSe2 (JCPDS #78-8410), and the good fit between the observed and calculated data in Fig. 1(b) further confirms the single phase nature of the sample according to the typical R values (Rp = 8.9%, Rwp = 10.32%) and goodness of fit (χ2 = 1.417).
 |
| | Fig. 1 XRD patterns of the products synthesized using hot injection method with different amounts of TETA after reaction at 250 °C for 1 h (a) and Rietveld refinement result of the sample synthesized with 200 μl TETA (b). | |
Fig. 2 shows the SEM micrographs of the products synthesized with various amounts of TETA. The product synthesized without addition of TETA appears to exhibit a mixture of rod-like crystallites with width of about 300 nm and lengths of 1–3 μm and irregular crystallites. The EDS spectrum of the rod-like crystallite and irregular crystallite indicates the relative ratio of copper, antimony, and selenium is about Sb2Se3 and Cu3SbSe4, respectively, agreeing with XRD result. For the product synthesized with 100 μl TETA, a mixture of rod-like crystallites and agglomerates with irregular shape was observed. As the TETA addition was increased to above 200 μl, the rod-like crystallites disappeared and the morphology became irregular shaped. The TEM micrograph of the product synthesized with 200 μl TETA is shown in Fig. 3. It indicates that the product synthesized with 200 μl TETA exhibits agglomerates consisting of sub-rounded crystallites with size of about 0.2 μm and short rod-like crystallites with width of about 200 nm and lengths of 200–500 nm. The EDS results (Table 1) suggest that the chemical composition in the crystallites is homogeneous and the relative ratio of copper, antimony, and selenium is nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, indicating the crystalline phase is CuSbSe2, which is consistent with XRD result.
2, indicating the crystalline phase is CuSbSe2, which is consistent with XRD result.
 |
| | Fig. 2 SEM micrographs of the products synthesized with various amounts of TETA (a) 0 μl, (b) 100 μl, (c) 200 μl and (d) 400 μl. | |
 |
| | Fig. 3 TEM micrograph of the product synthesized with 200 μl TETA. | |
Table 1 Chemical composition in Fig. 3 determined by EDS
| Composition (at%) |
Dot A |
Dot B |
| Cu |
23.92 |
23.99 |
| Sb |
26.70 |
27.85 |
| Se |
49.38 |
48.16 |
3.2 Influence of reaction time
Fig. 4 shows the XRD patterns of the products synthesized with 200 μl TETA after reacting for different periods. It indicates that Cu3SbSe4 and Sb2Se3 were the main phases, accompanied by a small amount of CuSbSe2 and residual selenium after reaction for 3 minutes. The diffraction peaks of the Cu3SbSe4 and Sb2Se3 phases gradually disappeared, but the diffraction peak intensity of CuSbSe2 phase increased with prolonging reaction time. As the reaction time was elongated to 30–60 minutes, the main crystalline phase became CuSbSe2. These suggest that Cu3SbSe4 may be the metastable phase and would be decomposed and converted into final stable CuSbSe2 as the system was continuously provided with energy.
 |
| | Fig. 4 XRD patterns of the products synthesized with 200 μl TETA after reacting for different periods. | |
Fig. 5 shows the scanning electron micrographs of the products synthesized with 200 μl TETA after reacting at 250 °C for different periods. For the product reacting for 3 minutes, a mixture of the spherical and needle crystallites was observed. Based on the EDS result, the needle crystallite chemical composition is close to Sb2Se3, hence, the needle crystallites can be identified as Sb2Se3. The EDS data of the atomic ratio of Cu/Sb for the spherical particle is close to 3, confirming the crystalline phase is Cu3SbSe4, which is consistent with the XRD result. The needle and spherical crystallites disappeared gradually with increasing reaction time. After reacting for 60 minutes, the product mainly consists of short rods with Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se of about 1
Se of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Based on the XRD and EDS results, the short-rod crystallites can be identified as CuSbSe2.
2. Based on the XRD and EDS results, the short-rod crystallites can be identified as CuSbSe2.
 |
| | Fig. 5 Scanning electron micrographs of the products synthesized with 200 μl TETA after reacting for different periods (a) 3 minutes, (b) 10 minutes, (c) 30 minutes and (d) 60 minutes. | |
TEM micrographs of the product obtained at 250 °C for 3 minutes are shown in Fig. 6. The EDS data shown in Table 2 confirms that the chemical compositions of the sub-rounded crystallite (Fig. 6(a)) and needle crystallite (Fig. 6(b)) are close to Cu3SbSe4 and Sb2Se3, respectively. As demonstrated in Fig. 7(a), the TEM diffraction spots of the sub-rounded crystallite can be indexed as (10![[5 with combining macron]](https://www.rsc.org/images/entities/char_0035_0304.gif) ), (11
), (11![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) and (
) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 16) reflections of Cu3SbSe4 (JCPDS #85-0003). The selected diffraction patterns of the needle crystallite can be indexed as (2
16) reflections of Cu3SbSe4 (JCPDS #85-0003). The selected diffraction patterns of the needle crystallite can be indexed as (2![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0), (3
0), (3![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), (400), (110) and (040) (Fig. 7(b)), confirming the needle crystallite is Sb2Se3.
0), (400), (110) and (040) (Fig. 7(b)), confirming the needle crystallite is Sb2Se3.
 |
| | Fig. 6 TEM micrographs of the product obtained at 250 °C for 3 minutes. | |
Table 2 Chemical compositions of the sub-rounded crystallite and needle crystallite (Fig. 6) determined by EDS
| Composition (at%) |
A |
B |
C |
D |
E |
| Cu |
35.53 |
39.59 |
36.92 |
8.45 |
7.00 |
| Sb |
13.25 |
12.96 |
11.59 |
38.16 |
38.86 |
| Se |
51.22 |
47.45 |
51.49 |
53.39 |
54.14 |
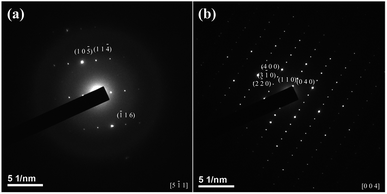 |
| | Fig. 7 TEM diffraction patterns of the sub-rounded crystallite (a) and needle crystallite (b) in Fig. 6. | |
Fig. 8 shows the TEM micrographs of the product obtained after reacting for 10 minutes. The chemical compositions of dots A–D in Fig. 8 are shown in Table 3. A large amount of copper ions have dissolved into the needle crystallites (dots A–C, Table 3). The chemical composition of the sub-rounded particle (dot D, Table 3) is close to CuSbSe2.
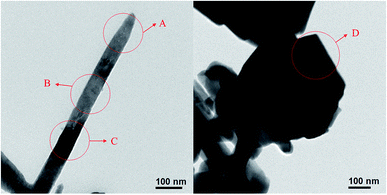 |
| | Fig. 8 TEM micrographs of the product obtained after reacting for 10 minutes. | |
Table 3 Chemical compositions in Fig. 8 determined by EDS
| Composition (at%) |
A |
B |
C |
D |
| Cu |
10.76 |
25.70 |
11.59 |
28.14 |
| Sb |
34.16 |
27.13 |
35.44 |
24.49 |
| Se |
55.08 |
49.16 |
52.97 |
47.37 |
A TEM micrograph of the crystallites obtained at 250 °C for 30 minutes is shown in Fig. 9. The powder morphology is short-rod and the particle size is between 50 and 200 nm. The EDS data shown in Table 4 confirms that the crystallite chemical composition is close to CuSbSe2.
 |
| | Fig. 9 TEM micrograph of the crystallites obtained at 250 °C for 30 minutes. | |
Table 4 Chemical compositions in Fig. 9 determined by EDS
| Composition (at%) |
A |
B |
C |
D |
| Cu |
29.86 |
28.91 |
28.81 |
23.89 |
| Sb |
25.45 |
24.97 |
24.14 |
23.02 |
| Se |
44.69 |
46.12 |
47.05 |
53.10 |
A TEM micrograph of the crystallites obtained at 250 °C for 60 minutes is shown in Fig. 10. The powder morphology is short-rod and the particle size is between 50 and 200 nm. The EDS data shown in Table 5 confirms that the chemical composition is close to CuSbSe2. As demonstrated in Fig. 11(a), the TEM diffraction spots can be indexed as (10![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ), (111) and (
), (111) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 15) reflections of CuSbSe2. Clear lattice fringes with interplanar spacing of 0.349 nm and 0.487 nm corresponding to (111) and (10
15) reflections of CuSbSe2. Clear lattice fringes with interplanar spacing of 0.349 nm and 0.487 nm corresponding to (111) and (10![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) planes of CuSbSe2 are displayed in Fig. 11(b). Based on the XRD, TEM and EDS results, the crystallites can be confirmed as CuSbSe2.
) planes of CuSbSe2 are displayed in Fig. 11(b). Based on the XRD, TEM and EDS results, the crystallites can be confirmed as CuSbSe2.
 |
| | Fig. 10 TEM micrograph of the crystallites obtained at 250 °C for 60 minutes. | |
Table 5 Chemical compositions in Fig. 10 determined by EDS
| Composition (at%) |
A |
B |
C |
| Cu |
24.33 |
25.77 |
23.91 |
| Sb |
26.74 |
25.37 |
26.70 |
| Se |
48.93 |
48.86 |
49.38 |
 |
| | Fig. 11 TEM diffraction and HRTEM lattice fringe of the crystallites obtained at 250 °C for 60 minutes. | |
XPS was employed to characterize the valence state of the elements in the product. Fig. 12 shows the XPS Cu 2p spectra of the products synthesized at 250 °C for different reaction time. The binding energies of Cu 2p3/2 and Cu 2p1/2 at about 933.70 eV and 953.41 eV can be observed in the product synthesized within 3 minutes, indicating that Cu2+ existed.33 The binding energies of Cu 2p3/2 at about 933 eV and 932 eV observed in the products synthesized within 30 minutes can be attributed to Cu2+ to Cu+, respectively, indicating that both Cu+ and Cu2+ existed.33 The ratio of Cu+ and Cu2+ increased from 1.68 to 12.7 as the reaction time was increased from 3 minutes to 30 minutes (Table 6), which may be due to the reducibility increased with increasing reaction time. As the reaction time was increased to 60 minutes, XPS spectrum showed a Cu 2p doublet at the binding energies of 932.1 eV (2p3/2) and 951.87 eV (2p1/2) with a separation of 19.77 eV, which is in agreement with Cu+, reported for CuSbS2.34 Even after reacting for 30 minutes, weak Cu2+ peaks still exist. Based on the results of XRD, XPS and TEM/EDS results, it may conclude that Cu+ and Cu2+ coexisted in Cu3SbSe4, which is in agreement with the previous literatures.35 Antimony XPS spectra for all products (Fig. 13) show a 3d doublet at the binding energy of 528.9 eV (3d5/2) and 538 eV (3d3/2) with a separation of 9.4 eV, which are consistent with Sb3+.22 The satellite peaks at the binding energy of near 530 eV and 531 eV for Sb 3d may result from the slight oxidation of Sb at the surface of crystallites. The O 1s peaks overlap with Sb 3d peak and correspond to the Sb–O bonding oxygen and the adsorbed oxygen, respectively, at lower and higher bonding energy.22 Moreover, the peaks at 35.5 eV (Sb 4d5/2) and 36.7 eV (Sb 4d3/2) for Sb5+ (ref. 34) were not found for all products. These results suggest that the valence state of Sb for all products is +3.
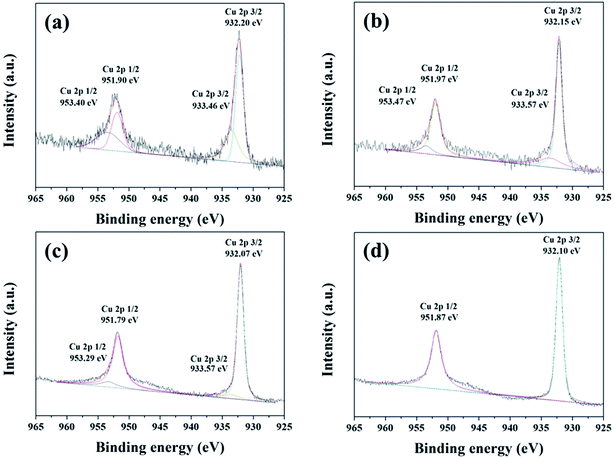 |
| | Fig. 12 XPS Cu 2p spectra of the products synthesized at 250 °C for different reaction time (a) 3 minutes, (b) 10 minutes, (c) 30 minutes and (d) 60 minutes. | |
Table 6 Ratios between the integrated area of XPS peaks of Cu+ and Cu2+ for the samples synthesized at 250 °C for different reaction time
| Time (minutes) |
3 |
10 |
30 |
60 |
| Cu+/Cu2+ ratio |
1.68 |
3.23 |
12.7 |
No Cu2+ |
 |
| | Fig. 13 Antimony XPS spectra of the products synthesized at 250 °C for different reaction time (a) 3 minutes, (b) 10 minutes, (c) 30 minutes and (d) 60 minutes. | |
Selenium XPS spectra (Fig. 14) shows that Se 3d5/2 peak at 53.6 eV and 3d3/2 peak at 54.5 eV with a separation of 0.9 eV, which are consistent with Se2−.36,37
 |
| | Fig. 14 Selenium XPS spectra of the products synthesized at 250 °C for different reaction time (a) 3 minutes, (b) 10 minutes, (c) 30 minutes and (d) 60 minutes. | |
Fig. 15 shows the Raman spectra of the products synthesized with 200 μl TETA after reaction for different times. The peaks at 259 cm−1, characteristic of binary copper selenides crystalline phases such as CuSe or Cu2−xSe are not observed.38 The vibrational mode at around 150–250 cm−1 can be assigned as the stretching of CuSe4 or SbSe4.39 After reaction for 3 minutes, two main broaden peaks located at 186 and 249 cm−1 were observed, which can be assigned to the peaks of Sb2Se3 and Cu3SbSe4 phases.39,40 The characteristic Raman peaks of Cu3SbSe4 overlap seriously with those of Sb2Se3, resulting in difficulty to discriminate between the two phases. The full width at half maximum (FWHM) of the peaks at about 190 and 250 cm−1 became narrow and the peaks shifted toward higher wave number with increasing reaction time. The Raman peak at about 210 cm−1 corresponding to Ag mode of CuSbSe2 phase appeared after reaction for 10 minutes and became progressively stronger in intensity with further increasing reaction time.41 This Raman response evolution indicates that a phase transition from Sb2Se3 and Cu3SbSe4 to chalcostibite CuSbSe2 occurs, which is in agreement with the XRD result.
 |
| | Fig. 15 Raman spectra of the products synthesized with 200 μl TETA after reaction for different times. | |
3.3 Formation mechanism of CuSbSe2
In the hot injection process for CuSbSe2 using TEG as the solvent, the TETA addition plays a key role in determining the final phase. After injection of cationic precursors and before TETA injection, sub-rounded Cu3SbSe4 and needle Sb2Se3 crystallites formed immediately (step 1). The phase separation may be due to the difference in the reactivity of precursors, Cu–TEG and Sb–TEG. Moreover, based on the XPS, XRD and TEM results, the presence of the intermediate phase, Cu3SbSe4 phase could be due to the existence of Cu2+, resulting from the insufficient reducibility in the solution. A sufficient amount of TETA acting as a reducing agent due to the presence of –NH2 groups can facilitate the reduction of Cu2+ in Cu3SbSe4 into Cu+, leading to the preformed Cu3SbSe4 phase dissolved and reacted with Sb2Se3 to form CuSbSe2 (step 2). The short-rod CuSbSe2 may be formed by the decomposition of needle Sb2Se3 resulting from the inward diffusion of Cu+, which will be studied in detail in the future. The schematic diagram of CuSbSe2 formation mechanism is shown in Fig. 16.
 |
| | Fig. 16 Schematic diagram of CuSbSe2 formation mechanism. | |
3.4 Optical absorption spectrum of CuSbSe2
The optical absorption spectrum of CuSbSe2 phase synthesized using 200 μl TETA after reacting at 250 °C for 60 minutes is shown in Fig. 17. The extrapolation of the linear section of the (αhν)2 versus hν plot for CuSbSe2 powder reveals a direct band gap with the band gap value estimated to be 1.06 eV, which agrees well with literature value.8
 |
| | Fig. 17 Optical absorption spectrum of CuSbSe2 phase synthesized using 200 μl TETA after reacting at 250 °C for 60 minutes. | |
4. Conclusions
A single phase CuSbSe2 crystallites can be obtained by using triethylene glycol as the solvent and reducing agent and triethylenetetramine as co-reducing agent. Without addition of TETA, Cu3SbSe4 and Sb2Se3 were the main phases and no CuSbSe2 phase was observed. XPS results indicate that the valence states of copper for CuSbSe2 and Cu3SbSe4 may be +1 and a mix of +1 and +2, respectively. A sufficient amount of TETA can facilitate the reduction of Cu2+ into Cu+, leading to the preformed Cu3SbSe4 phase dissolved and reacted with Sb2Se3 to form CuSbSe2. The obtained CuSbSe2 phase had a direct band gap with the band gap value of 1.06 eV.
Acknowledgements
This work was financially sponsored by the Ministry of Science and Technology, Republic of China (103-2623-E-006-011-ET).
References
- B. Koo, R. N. Patel and B. A. Korgel, J. Am. Chem. Soc., 2009, 131, 3134 CrossRef CAS PubMed.
- S. J. Ahn, K. H. Kim and K. H. Yoon, Colloids Surf., A, 2008, 313–314, 171 CrossRef.
- J. Xiao, Y. Xie, Y. Xiong, R. Tang and Y. Qian, J. Mater. Chem., 2001, 1417 RSC.
- J. Gifford, PV Mag, 2015, http://www.pv-magazine.com/news/details/beitrag/solar-frontier-hits-223-on-cigscell_100022342/#axzz45roL5wnL Search PubMed.
- J.-F. Guillemoles, L. Kronik, D. Cahen, U. Rau, A. Jasenek and H.-W. Schock, J. Phys. Chem. B, 2000, 104, 4849 CrossRef CAS.
- Z. Ning, Z. Da-Ming and Z. Gong, Mater. Sci. Eng., B, 2010, 166, 34 CrossRef.
- C. Candelise, M. Winskel and R. Gross, Prog. Photovolt: Res. Appl., 2012, 20, 816 CrossRef.
- K. Takei, T. Maeda and T. Wada, Thin Solid Films, 2015, 582, 263 CrossRef CAS.
- J. Zhou, G. Q. Bian, Q. Y. Zhu, Y. Zhang, C. Y. Li and J. Dai, J. Solid State Chem., 2009, 182, 259 CrossRef CAS.
- D. Tang, J. Yang, F.-Y. Liu, Y.-Q. Lai, J. Li and Y.-X. Liu, Electrochim. Acta, 2012, 76, 480 CrossRef CAS.
- S. Manolache, A. Duta, L. Isac, M. Nanu, A. Goossens and J. Schoonman, Thin Solid Films, 2007, 515, 5957 CrossRef CAS.
- Y. Rodríguez-Lazcano, M. T. S. Nair and P. K. Nair, J. Electrochem. Soc., 2005, 152, G635 CrossRef.
- S. Banua, S. J. Ahna, S. K. Ahn, K. Yoon and A. Cho, Sol. Energy Mater. Sol. Cells, 2016, 151, 14 CrossRef.
- K. Ramasamy, H. Sims, W. H. Butler and A. Gupta, Chem. Mater., 2014, 26, 2891 CrossRef CAS.
- W. H. Hsu, H. I. Hsiang, C. T. Chia and F. S. Yen, J. Solid State Chem., 2013, 208, 1 CrossRef CAS.
- M. Y. Chiang, S. H. Chang, C. Y. Chen, F. W. Yuan and H. Y. Tuan, J. Phys. Chem. C, 2011, 115, 1592 CAS.
- W. H. Hsu, H. I. Hsiang, Y. L. Chang, D. T. Ray and F. S. Yen, J. Am. Ceram. Soc., 2011, 94, 3030 CrossRef CAS.
- K. Ramasamy, H. Sims, W. H. Butler and A. Gupta, J. Am. Chem. Soc., 2014, 136, 1587 CrossRef CAS PubMed.
- C. Yan, Z. Su, E. Gu, T. Cao, J. Yang, J. Liu, F. Liu, Y. Lai, J. Li and Y. Liu, RSC Adv., 2012, 2, 10481 RSC.
- Y. Liu, J. Yang, E. Gu, T. Cao, Z. Su, L. Jiang, C. Yan, X. Hao, F. Liu and Y. Liu, J. Mater. Chem. A, 2014, 2, 6363 CAS.
- S. Ikeda, S. Sogawa, Y. Tokai, W. Septina, T. Harada and M. Matsumura, RSC Adv., 2014, 4, 40969 RSC.
- X. Qiu, S. Ji, C. Chen, G. Liu and C. Ye, CrystEngComm, 2013, 15, 10431 RSC.
- C. N. Bucheri, K. R. Oleson and H. W. Hillhouse, Curr. Opin. Chem. Eng., 2013, 2, 168 CrossRef.
- J. J. Wang, Y. Q. Wang, F. F. Cao, Y. G. Guo and L. J. Wan, J. Am. Chem. Soc., 2010, 132, 12218 CrossRef CAS PubMed.
- T. Liu, Z. Jin, J. Li, J. Wang, D. Wang, J. Lai and H. Du, CrystEngComm, 2013, 15, 8903 RSC.
- T. Wang, Z. G. Jin, T. J. Liu, W. L. Li and Y. Ni, J. Am. Ceram. Soc., 2010, 93, 1927 CAS.
- H. Liu, Z. G. Jin, W. D. Wang and J. Li, CrystEngComm, 2011, 13, 7198 RSC.
- J. Xu, C. Y. Luan, Y. B. Tang, X. Chen, J. A. Zapien, W. J. Zhang, H. L. Kwong, X. M. Meng, S. T. Lee and C. S. Lee, ACS Nano, 2010, 4, 6064 CrossRef CAS PubMed.
- J. Li, Z. Jin, T. Liu, J. Wang, X. Zheng and J. Lai, CrystEngComm, 2014, 16, 6819 RSC.
- M. R. Mohammadi, V. Zarghami and D. J. Fray, Appl. Phys. A: Mater. Sci. Process., 2012, 107, 497 CrossRef CAS.
- J. Yang, C. Zang, G. Wang, G. Xu and X. Cheng, J. Alloys Compd., 2010, 495, 158 CrossRef CAS.
- J. Chang, J. E. Han and D.-Y. Jung, Bull. Korean Chem. Soc., 2011, 32, 434 CrossRef CAS.
- X. Q. Chen, Z. Li and S. X. Dou, ACS Appl. Mater. Interfaces, 2015, 7, 13295 CAS.
- B. Yang, L. Wang, J. Han, Y. Zhou, H. Song, S. Chen, J. Zhong, L. Lv, D. Niu and J. Tang, Chem. Mater., 2014, 26, 3135–3143 CrossRef CAS.
- V. B. Ghanwat, S. S. Mali, R. M. Mane, P. S. Patil, C. K. Hong and P. N. Bhosale, New J. Chem., 2015, 39, 5661 RSC.
- B. Canava, J. Vigneron, A. Etcheberry, J. F. Guillemoles and D. Lincot, Appl. Surf. Sci., 2002, 202, 8 CrossRef CAS.
- J. Lauth, F. E. S. Gorris, M. S. Khoshkhoo, T. Chassé, W. Friedrich, V. Lebedeva, A. Meyer, C. Klinke, A. Kornowski, M. Scheele and H. Weller, Chem. Mater., 2016, 28, 1728 CrossRef CAS.
- J. H. Shi, Z. Q. Li, D. W. Zhang, Q. Q. Liu, Z. Sun and S. M. Huang, Prog. Photovoltaics, 2011, 19, 160 CAS.
- R. B. Balow, E. P. Tomlinson, M. M. Abu-Omar, B. W. Boudouris and R. Agrawal, J. Mater. Chem. A, 2016, 4, 2198 CAS.
- T. Y. Ko and K. W. Sun, J. Lumin., 2009, 129, 1747 CrossRef CAS.
- J. Baker, R. S. Kumar, D. Sneed, A. Connolly, Y. Zhang, N. Velisavljevic, J. Paladugu, M. Pravica, C. Chen, A. Cornelius and Y. Zhao, J. Alloys Compd., 2015, 643, 186 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *,
Chang-Ting Yang and
Jui-Huan Tu
*,
Chang-Ting Yang and
Jui-Huan Tu
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, indicating the crystalline phase is CuSbSe2, which is consistent with XRD result.
2, indicating the crystalline phase is CuSbSe2, which is consistent with XRD result.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sb
Sb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se of about 1
Se of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Based on the XRD and EDS results, the short-rod crystallites can be identified as CuSbSe2.
2. Based on the XRD and EDS results, the short-rod crystallites can be identified as CuSbSe2.
![[5 with combining macron]](https://www.rsc.org/images/entities/char_0035_0304.gif) ), (11
), (11![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) and (
) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 16) reflections of Cu3SbSe4 (JCPDS #85-0003). The selected diffraction patterns of the needle crystallite can be indexed as (2
16) reflections of Cu3SbSe4 (JCPDS #85-0003). The selected diffraction patterns of the needle crystallite can be indexed as (2![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0), (3
0), (3![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0), (400), (110) and (040) (Fig. 7(b)), confirming the needle crystallite is Sb2Se3.
0), (400), (110) and (040) (Fig. 7(b)), confirming the needle crystallite is Sb2Se3.
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ), (111) and (
), (111) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 15) reflections of CuSbSe2. Clear lattice fringes with interplanar spacing of 0.349 nm and 0.487 nm corresponding to (111) and (10
15) reflections of CuSbSe2. Clear lattice fringes with interplanar spacing of 0.349 nm and 0.487 nm corresponding to (111) and (10![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) planes of CuSbSe2 are displayed in Fig. 11(b). Based on the XRD, TEM and EDS results, the crystallites can be confirmed as CuSbSe2.
) planes of CuSbSe2 are displayed in Fig. 11(b). Based on the XRD, TEM and EDS results, the crystallites can be confirmed as CuSbSe2.












