Upconversion nanoparticle arrays for detecting glycated hemoglobin with high sensitivity and good reusability†
Pan Hua,
Xiaofeng Wu*a,
Shigang Hua,
Zhijun Tanga,
Gangtao Daib and
Yunxin Liu*bc
aSchool of Information and Electrical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China. E-mail: xfwuvip@126.com
bDepartment of Physics and Electrical Science, Hunan University of Science and Technology, Xiangtan 411201, China. E-mail: lyunxin@163.com
cINPAC-Institute for Nanoscale Physics and Chemistry, KU Leuven, Celestijnenlaan 200D B-3001, Belgium
First published on 21st October 2016
Abstract
Lanthanide-doped upconversion nanoparticles (UCNPs) have attracted extensive interest in bio-applications due to their unique optical properties by converting near infrared excitation to visible emission. Here, we show that NaGdF4:48% Yb3+,2% Er3+ upconversion nanoparticle arrays could be used as a high-sensitive, reusable, and stable device for detecting glycosylated hemoglobin (HbA1c), based on the luminescent resonance energy transfer (LRET) from UCNPs to HbA1c. Importantly, the upconversion nanoparticle arrays present a much higher sensitivity to the concentration variation of HbA1c than the direct detection in the solution, which is ascribed to the extinction effect of the arrays.
1. Introduction
Glycated hemoglobin (HbA1c) is a stable glucose adduct with the N-terminal valine of the β-chain of haemoglobin,1,2 the level of which indicates the average blood glucose level over the previous 2 to 3 months, and is currently a very useful diagnostic index and allows long-term monitoring for diabetes.3–6 Conventional detections of HbA1c are usually determined via electrochemical biosensors, because of their low cost, good sensitivity, and small size.7–9 However, they require electrode modification, separation of total hemoglobin content, and fabrication of the sensing interface involving stepwise attachment of a molecular wire, redox species, and an HbA1c analogue for competitive inhibition.10,11 Hence, it is desirable to develop an easy-to-use and also highly sensitive biosensor for detecting HbA1c.12,13UCNPs are inorganic lanthanide-doped nanocrystals, capable of converting lower-energy infrared photons into higher-energy visible light emission.14–19 Because of the infrared light being used as the excitation power source, UCNPs generate the expected fluorescent signals without background.20–23 In addition, the upconversion luminescence has the following characteristics: good photo-stability, narrow emission bands, exciting with cost-effective infrared laser diode.24–27
Kim et al. have recently reported a viable and efficient procedure for detecting HbA1c through luminescent resonance energy transfer process from upconversion nanoparticles to HbA1c.1 Their results indicated that the concentration of HbA1c can be easily addressed according to the luminescence intensity of UCNPs, since the luminescence intensity decreases monotonously with increasing the concentration of HbA1c.
Here, we show that UCNPs arrays show higher sensitivity to the concentration variation of HbA1c than the direct detection in solution. In addition, this array sensor is reusable and the detection data is highly reproducible, due to a fact that the instability of UCNPs in solution is avoided.
2. Experimental
UCNPs were synthesized by the previous route28 (details listed in ESI†). Subsequently, the surfaces of these UCNPs were modified with PEG according to previous protocols with slight modification.29,30 The fabrication of the UCNPs arrays was illustrated in Scheme S1 (see ESI† for the details). The morphology and particle size of the synthesized nanoparticles were characterized by JEM 3010 high-resolution transmission electron microscopy (HRTEM). The UV-vis absorption spectra of HbA1c was recorded on a Shimadzu UV-2550 spectrometer. The photoluminescence spectra were measured by Hitachi F-2700 fluorescence spectrophotometer while the fluorescent photographs were taken on Olympus BX43 inverted fluorescence microscope.3. Results and discussion
3.1 Upconversion fluorescence of NaGdF4 nanoparticles
By using a facile strategy, NaGdF4:48% Yb3+,2% Er3+ upconversion nanoparticles with efficient photoluminescence and uniform particle size were successfully prepared. The morphology and particle size of the synthesized NaGdF4:48% Yb3+,2% Er3+ nanocrystals can be addressed by TEM and HRTEM images in Fig. 1. It is clear (Fig. 1a) that the as-prepared nanoparticles appear almost spherical in shape and monodisperse. The average diameters of these prepared NaGdF4 nanoparticles doped with 48% Yb3+ and 2% Er3+ are determined to be about 28.5 ± 0.5 nm. The crystal lattice fringes (Fig. 1b) were clearly observed along (0001) orientation in the hexagonal NaGdF4 nanocrystals.31 | ||
| Fig. 1 (a) TEM image of NaGdF4:48% Yb3+,2% Er3+ NPs; (b) the corresponding high-resolution TEM image (HR-TEM). | ||
The upconversion fluorescent spectra of NaGdF4:48% Yb3+,2% Er3+ nanocrystals in cyclohexane solution under the 980 nm laser excitation are shown in Fig. 2. The emission bands can be easily assigned to transition within the 4f–4f levels of the Er3+. The spectrum of NaGdF4:48% Yb3+,2% Er3+ exhibits three distinct emission bands from Er3+ ions, centered at 521 nm, 540 nm, and 654 nm which are assigned to the Er3+–4fn electronic transitions 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2, respectively. The whole luminescence appears green-yellow in color to naked eyes due to the combination of intense green and weak red emissions from the Er3+ ion.
The possible upconversion excitation emission pathways of the Er3+/Yb3+ ion couples are shown in Fig. S1.† In the case of NaGdF4:48% Yb3+,2% Er3+ nanocrystals, an initial energy transfer from an Yb3+ ion in the 2F5/2 state to an Er3+ ion populating the 4I11/2 level of Er3+ ion. The second 980 nm photon or energy transfer from an Yb3+ ion to the Er3+ ion can then populate the 4F7/2 level of the Er3+ ion. The electrons in the 4F7/2 level of Er3+ ion can relax nonradiatively (without emission of photons) to the 4S3/2 levels for the green emissions by the transition 4S3/2 → 4I15/2. Alternatively, the Er3+ ion can further relax and populate the 4F9/2 level leading to the red emission from the transition 4F9/2 → 4I15/2. The 4F9/2 level may also be populated from the 4I13/2 level of the Er3+ ion by absorption of a 980 nm photon, or energy transfer from an Yb3+ ion, with the 4I13/2 state being initially populated via the nonradiative 4I11/2 → 4I13/2 relaxation.32
3.2 LRET mechanism and the direct detection of glycated hemoglobin in solution
The luminescent resonance energy transfer (LRET) mechanism has been reported in previous works.1,29,33 Here, NaGdF4:48% Yb3+,2% Er3+ nanoparticles play a role of the fluorescence donor while HbA1c performs as an acceptor. As we can see from Fig. 3, the green emission spectrum of the UCNPs ranging from 510 to 570 nm suitably overlaps with the absorption spectrum of HbA1c. Therefore, the green luminescence of the UCNPs can be strongly quenched by HbA1c. | ||
| Fig. 3 The UC fluorescence spectrum of NaGdF4:48% Yb3+,2% Er3+ nanocrystals and the absorption spectrum of HbA1c between 510 nm and 570 nm. | ||
HbA1c is a stable glucose adduct, of which the level indicates the average blood glucose level over the previous 2 to 3 months. There is no change to the level of HbA1c with the daily fluctuation in the blood, and is currently a very useful diagnostic index and allows long-term monitoring for diabetes. Conventional detection of HbA1c is usually determined via electrochemical biosensors, because of their low cost, high sensitivity, and small size. However, they require electrode modification, separation of total hemoglobin content, and fabrication of the sensing interface involving stepwise attachment of a molecular wire, redox species, and an HbA1c analogue for competitive inhibition. Hence, there is a need to develop a novel homogeneous biosensor for HbA1c detection with high selectivity and simplicity. Here, we show that the green upconversion fluorescent nanoprobes is very efficient and viable for detecting HbA1c, based on the luminescent resonance energy transfer process from UCNPs to HbA1c. The main merits of these UCNPs based LRET are ascribed to the deep penetration depth of the infrared excitation light and high signal to noise ratios. This efficient LRET process between UCNPs and HbA1c could be directly used for detecting the concentration of HbA1c in solution.
3.3 Detection of glycated hemoglobin by upconversion nanoparticle arrays and the enhanced sensitivity
We have directly detected HbA1c in solution (see Fig. S5 and the related discussion in ESI†) and found that the repeatedly measured fluorescent spectra have a deviation of 3–12% in intensity relative to the original one, due to the fact that the solution containing UCNPs (16.5 mg ml−1) and HbA1c is not completely uniform and stable and a small part of UCNPs deposit gradually in the solution during the detecting process. In addition, the particular processing procedure, such as the surface modification of UCNPs and shaking, is necessary to ensure the solution as uniform as possible.To overcome these problems involved in the direct detection of HbA1c in solution, UCNPs arrays were developed for detecting HbA1c with higher sensitivity to the concentration variation (∼four times of that in solution), better reproducibility of the fluorescent signals (deviation of 0.4–1.8%), and good reusability (repeated 20 times without obvious deviation).
UCNPs were first synthesized by the solvothermal method in the OA/ODE solution, and then modified with PEG to transfer their surfaces from hydrophobic to hydrophilic. These nanoparticles were subsequently washed with distilled water for several times to remove excess PEG. As demonstrated in Scheme S1,† these PEG modified UCNPs were deposited in the holes of the array board (Fig. 4a) with close two-dimensional structures due to the solid state PEG involved in the linking among nanoparticles. It should be emphasized that the PEG coating with the thickness of several nanometers could exist stably on the surfaces of UCNPs and would not resolve in water during the detection process.29 The UCNPs arrays could emit green light under the excitation of 980 infrared light (Fig. 4b).
 | ||
| Fig. 4 (a) The bright field imaging of the UCNPs array and (b) fluorescence imaging of the UCNPs array under the excitation of a 980 nm laser diode. | ||
The detection based on these arrays is a promising way to improve the sensitivity. We get one drop of glycated hemoglobin solution on the array, and then a series of LRET spectra were recorded (Scheme 1) and shown in Fig. 5. It is noted that the integral emission intensity from the array decreases remarkably with increasing the content of HbA1c. The sensitivity (S) is defined as the follows:
| S = (Ij − Ij+1)/Ij |
 | ||
| Fig. 6 The detection sensitivity (S) to the concentration variation of HbA1c in solution and on array. | ||
3.4 The mechanism involved in the detection based on the arrays and the reusability and reproducibility
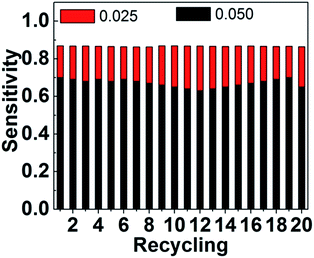
For demonstrating the difference between the detections in solution and on array, the radiation radius is adopted for representing the emission intensity (Scheme 2). When detecting directly HbA1c in solution, the luminescence of UCNPs has a certain radiation radius and becomes shorter with increasing the concentration of HbA1c (Scheme 2a). However, for detection on the array, the collected photons are actually attributed to the emission overlap from the luminous spots on the array (Scheme 2b). It should be noted that the luminescent intensity of the overlapping region deceases much faster than the emission centers, with increasing the concentration of HbA1c. e.g., the emission from the overlapping region will vanish, while the emission intensity of emission centers decreases to half of the original one (Scheme 2b). Of course, this emission overlap obeys the laws of geometrical optics. Therefore, it can be easily inferred that the collected photons on the array are more sensitive to the concentration variation of HbA1c than those in the solution. In addition, the upconversion nanoparticles were deposited on the array and formed a close film to ensure the stability of detection process and the good reproducibility of detection data.The further investigation indicates that the ever-used UCNPs arrays can be cleaned with the mixture of ethanol and water (Scheme 1) and used for detecting HbA1c again. There is no obvious deviation for the detection results after repeating for 20 times (Fig. 7).
4. Conclusions
Upconversion nanoparticle (NaGdF4:48% Yb3+,2% Er3+) arrays were designed and fabricated, which can be used for detecting HbA1c through the LRET from UCNPs to HbA1c. The extinction effect of the UCNPs arrays contributed to the enhancement of the detection sensitivity. In comparison to the direct detection in solution, this method presents higher sensitivity to the concentration variation (∼4 times of that in solution), better reproducibility of the fluorescent signals (deviation: 0.4–1.8% on array; 3–12% in solution), and good reusability (repeated 20 times without obvious deviation). This method can be used for detecting glycated and non-glycated hemoglobin, while the selective detection of them is under way.We expect these UCNPs arrays will accelerate the application of biosensors based on UCNPs in the field of biological detections.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant No. 61674056, 61675067 and 61575062); supported by the Scientific Research Fund of Hunan Provincial Education Department (Grant No. 16A072 and 16C0627).Notes and references
- E. W. Jo, H. Mun and M. G. Kim, Anal. Chem., 2016, 88, 2742 CrossRef CAS PubMed.
- W. Hoelzed, S. Weykamp, J. Jeppsson, K. Miedema, W. G. John and H. Wiedmeyer, et al., Clin. Chem., 2004, 50, 1166 Search PubMed.
- C. S. Pundir and S. Chawla, Anal. Biochem., 2014, 444, 47 CrossRef CAS PubMed.
- W. G. John, Clin. Chem. Lab. Med., 2003, 41, 1199 CrossRef CAS PubMed.
- The Diabetes Control and Complications Trial Research Group, The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus, N. Engl. J. Med., 1993, 329, 977–986 CrossRef PubMed.
- J. O. Jeppsson, U. Kobold, J. Barr, A. Finke, W. Hoelzel, T. Hoshino, K. Miedema, A. Mosca, P. Mauri, R. Paroni, L. Thienpont, M. Umemoto and C. Weykamp, Clin. Chem. Lab. Med., 2002, 40, 78 CrossRef CAS PubMed.
- J. M. Lee, E. L. Wu, B. Tarini, W. H. Herman and E. J. Yoon, Pediatrie, 2011, 158, 947 CrossRef CAS PubMed.
- J. Pribyl and P. Skladal, Biosens. Bioelectron., 2006, 21, 1952 CrossRef CAS PubMed.
- D. B. Sacks, D. E. Bruns, D. E. Goldstein, N. K. Maclaren, J. M. Mcdonald and M. Parrott, Clin. Chem., 2002, 48, 436 CAS.
- M. Sheikholeslam, M. D. Pritzker and P. Chen, InTech, 2011, 42, 293 Search PubMed.
- W. V. Mukerjee, S. D. Collins, R. R. Isseroff and R. L. Smith, Sens. Actuators, A, 2004, 114, 267 CrossRef.
- G. Liu, S. M. Khor, S. G. Iyengar and J. J. Gooding, Analyst, 2012, 137, 829 RSC.
- Q. Xue, C. Bian, J. Tong, J. Sun, H. Zhang and S. Xia, Biosens. Bioelectron., 2011, 26, 2689 CrossRef CAS PubMed.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Chem. Rev., 2015, 115, 395 CrossRef CAS PubMed.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343 CrossRef CAS PubMed.
- Y. Liu, D. Tu, H. Zhu and X. Chen, Chem. Soc. Rev., 2013, 42, 6924 RSC.
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- H. S. Mader, P. Kele and S. M. Saleh, Curr. Opin. Chem. Biol., 2010, 14, 582 CrossRef CAS PubMed.
- W. Feng, L. D. Sun, Y. W. Zhang and C. H. Yan, Coord. Chem. Rev., 2010, 254, 1038 CrossRef CAS.
- P. Hu, X. Wu, S. Hu, Z. Chen and Y. Liu, Photochem. Photobiol. Sci., 2016, 15, 260 CAS.
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- J. Shen, L. D. Sun and C. H. Yan, Dalton Trans., 2008, 5687 RSC.
- X. Wu, P. Hu, S. Hu, Z. Chen and Y. Liu, J. Rare Earths, 2016, 34, 208 CrossRef CAS.
- F. Vetrone and J. A. Capobianco, Int. J. Nanotechnol., 2008, 5, 1306 CrossRef CAS.
- P. Rahman and M. Green, Nanoscale, 2009, 1, 214 RSC.
- F. Wang, D. Banerjee, Y. S. Liu, X. Y. Chen and X. G. Liu, Analyst, 2010, 135, 1839 RSC.
- M. Haase and H. Schafer, Angew. Chem., Int. Ed., 2011, 50, 5808 CrossRef CAS PubMed.
- Y. Liu, D. Wang, J. Shi, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2013, 52, 4366 CrossRef CAS PubMed.
- Z. Chen, X. Wu, S. Hu, P. Hu and Y. Liu, J. Mater. Chem. C, 2015, 3, 6067 RSC.
- M. Wang, G. Abbineni, A. Clevenger, C. B. Mao and S. K. Xu, Nanomedicine: Nanotechnology, Biology and Medicine, 2011, 7, 710 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–760 CrossRef.
- J. C. Boyer, L. A. Cuccia and J. A. Capobianco, Nano Lett., 2007, 7, 847 CrossRef CAS PubMed.
- Z. Chen, X. Wu, S. Hu, P. Hu and Y. Liu, J. Mater. Chem. C, 2015, 3, 153 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20642e |
| This journal is © The Royal Society of Chemistry 2016 |