Supramolecular porous ionic network based on triazinonide and imidazolium: a template-free synthesis of meso-/macroporous organic materials via a one-pot reaction-assembly procedure†
Abstract
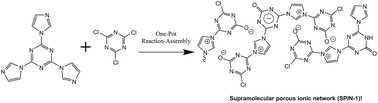
A supramolecular porous ionic network (SPIN-1) was designed and prepared using a one-pot procedure, involving the quaternization of triimidazole triazine with cyanuric chloride followed by hydrolysis and in situ assembly. The structure of SPIN-1 was characterized by FT-IR, solid-state 13C CP/TOSS NMR, elemental analysis and XPS. SPIN-1 shows a BET surface area up to 263 m2 g−1 (average pore size 11 nm), and a CO2 capture capacity of 24.7 cm3 g−1, which is competitive for Carbon Capture and Storage (CCS).

 Please wait while we load your content...
Please wait while we load your content...