Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts
Zhimin Xue*a,
Ming-Guo Maa,
Zhonghao Lib and
Tiancheng Mu*c
aBeijing Key Laboratory of Lignocellulosic Chemistry, College of Materials Science and Technology, Beijing Forestry University, Beijing 100083, China. E-mail: zmxue@bjfu.edu.cn
bMinistry of Education, Key Laboratory of Colloid & Interface Chemistry, Shandong University, Jinan 250100, China
cDepartment of Chemistry, Renmin University of China, Beijing 100872, China. E-mail: tcmu@ruc.edu.cn; Tel: +86-10-62514925
First published on 12th October 2016
Abstract
Efficient conversion of glucose and cellulose to 5-hydroxymethylfurfural (HMF) is one of the most successful and promising routes in the field of biomass transformation. In recent years, many kinds of catalysts have been developed for this important reaction, and much progress has been achieved. Compared with homogeneous catalysts, heterogeneous acidic catalysts have attracted much more interest for dehydration of glucose and cellulose to HMF due to their separable and reusable nature. This review focuses on efficient and selective conversion of glucose and cellulose to HMF catalyzed by heterogeneous catalysts in various solvent systems, including water, organic solvents, ionic liquids, mixed solvents, and biphasic systems. At the end of this review, an outlook is provided to highlight the challenges and opportunities related to this interesting and important route for HMF production from glucose and cellulose.
1. Introduction
The illogicality between the diminishing reserves of fossil resources and the increasing demands for energy and chemicals is a challenging problem faced by mankind. Therefore, the search for renewable alternative resources has become a global research interest in academia and industry.1–3 Among various renewable resources, cellulose, the most abundant component of lignocellulosic biomass,4 has attracted significant attention as a human-inedible renewable carbon resource for the selective production of various value-added chemicals and fuels,5–9 and a series of research and development plans on the utilization of cellulose have been launched by many countries.10,11In terms of cellulose transformation, catalytic dehydration of cellulose to produce 5-hydroxymethylfurfural (HMF) is one of the most promising and successful routes because HMF has been recognized as one of the top building block chemicals generated from biomass.12 As a well-known compound, HMF can be transformed into many versatile compounds currently derived from petroleum by employing various types of reactions (Fig. 1),13 including 2,5-furandicarboxylic acid14 and 2,5-diformylfuran15 from oxidations, 2,5-bishydroxymethylfuran16 and 2,5-dimethylfuran17 from selective hydrogenations, and C7–C15 liquid alkanes from aldol condensation subsequent with hydrogenation,18 levulinic acid from hydrolysis,19 etc.
 | ||
| Fig. 1 HMF as a platform chemical for diverse reactions. Reproduced from ref. 13 with permission from American Chemical Society. | ||
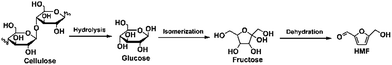
Generally, cellulose can be converted to HMF through a three-step reaction, involving hydrolysis of cellulose to glucose, isomerization of glucose to fructose, and dehydration of fructose to HMF (Fig. 2).20 Meanwhile, glucose can be easily obtained from the hydrolysis of cellulose,21,22 which is the first step in dehydration of cellulose to HMF. More importantly, the process for dehydration of glucose is the same as the last two steps of cellulose dehydration. Therefore, dehydration of glucose to HMF can be considered as an important component of cellulose dehydration, which is also included in this review. However, it should be pointed out that the more complex structure of cellulose results in lower solubility in most of solvents and difficulty to interact with the catalytic active sites. Therefore, conversion of cellulose often shows lower reaction rate and less HMF yield compared with glucose in the same catalytic system. In a long period of time, considerable interest has been focused on homogeneous acidic catalysts for HMF production from dehydration of glucose and cellulose.23–40 Although these homogeneous catalysts provided good catalytic performance, some drawbacks limited their practical applications, such as reactor corrosion, waste treatment and poor separation and/or recyclability. From the view point of practical applications and green chemistry, heterogeneous acidic catalysts should be paid more attention for dehydration of glucose and cellulose to HMF due to their separable and reusable nature, which can overcome the limitations of homogeneous catalyst to some extent. Therefore, more and more attentions have been devoted on the dehydration of glucose and cellulose into HMF over various types of heterogeneous acidic catalysts in a series of solvent systems, and much progress has been achieved in this attractive topic in recent years. However, during conversion of glucose and cellulose, some side reactions can be occurred under the acidic catalysts,41 such as the formation of humin or oligomers through the cross-polymerization between glucose and HMF or self-polymerization of HMF, rehydration of the HMF to levulinic acid and formic acid, and degradation of cellulose and glucose. Therefore, more efforts are being paid on the design efficient catalytic systems for conversion of glucose and cellulose to HMF to avoid or decrease the occurrence of the side reactions.
Although some reviews about transformation of carbohydrates to HMF have been reported,42–45 there is no one specialized in heterogeneous catalysts for conversion of glucose and cellulose to HMF, which is a more attractive field. More importantly, the field for HMF production from glucose and cellulose is developed very fast, and many progresses are continuously made. Therefore, a real-time and comprehensive review about heterogeneous conversion of glucose and cellulose to HMF is still needed.
In this review, we attempt to provide the state-of-the-art of catalytic dehydration of glucose and cellulose to HMF over heterogeneous acidic catalysts (i.e., metal oxides, zeolites, functional polymers, resins, carbonaceous catalysts, functionalized silicas, heteropoly acids, metal phosphates, immobilized ionic liquids, and some other special types of heterogeneous catalysts) in various solvent systems, including water, organic solvents, ionic liquids, mixed solvents, and biphasic systems. In addition, an outlook is provided to highlight the challenges and opportunities related to this interesting and important route for HMF production.
2. Heterogeneous dehydration of glucose and cellulose in water
From the view point of green chemistry, water is an ideal solvent because it is cheap, non-toxic, and non-flammable. Although many kinds of reactions can be conducted efficiently by using water as the reaction media,46–48 the yields of HMF from heterogeneous dehydration of glucose and cellulose are generally not satisfactory because of the unstable nature of HMF in water with the existence of acidic catalysts. To date, there are several successful examples for dehydration of glucose and cellulose over heterogeneous acidic catalysts in water, but the yields are generally low.2.1. Metal oxides for dehydration of glucose and cellulose in water
Metal oxides with porous structures are the most common used heterogeneous catalysts for dehydration of glucose and cellulose.The activity of TiO2 and ZrO2 for dehydration of glucose was firstly studied by Watanabe et al. in hot compressed water (473 K).49 Anatase TiO2 was found to act as an acidic catalyst to promote the formation of HMF from glucose with a yield of about 16% for 10 h, while ZrO2 was a basic catalyst to promote the isomerization of glucose to fructose. Through TPD measurement, they found that the amount of basic sites was the key factor for the isomerization while the density of acidity and basicity was important for the HMF formation from glucose.50 Following these two works, Qi et al. used TiO2 and ZrO2 as the heterogeneous catalyst for dehydration of glucose under microwave heating in hot compressed water.51 It was found that TiO2 and ZrO2 could promote the dehydration of glucose. At 473 K with a reaction time of 5 min, the yields of HMF could reach 18.6% and 10.0% over TiO2 and ZrO2, respectively (Table 1, entries 1–2). The authors suggested that ZrO2 acting as a base could promote the isomerization of glucose to fructose, while TiO2 could not only promote the isomerization of glucose to fructose but also enhance the HMF formation from glucose. Therefore, TiO2 showed a better performance than ZrO2.
| Entry | Substrate | Catalyst | Ta (K) | ta | Ca (%) | Ya (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a T = temperature; t = time; C = conversion; Y = HMF yield.b The amount of water was about 5 ml. With microwave heating.c TiO2–N-773 was obtained from the precipitation of TiO(NO3)2 calcined at 773 K; TiO2–Cl-773 was obtained from the precipitation of TiCl4 calcined at 773 K; ZrO2–N-773 was obtained from the precipitation of ZrO(NO3)2 calcined at 773 K; ZrO2–Cl-773 was obtained from the precipitation of ZrCl4 calcined at 773 K; SO4(1.8)–ZrO2-773 was the catalyst with 1.8% sulfur content calcined at 773 K. The amount of water was about 1 ml.d Bimodal-HZ-5 was prepared by post-synthesis modification of H-ZSM-5 with desilication. The amount of water was about 10 ml.e DS represented dodecyl sulfate (OSO3C12H25). The amount of water was about 4 ml. | |||||||
| 1b | Glucose (0.1 g) | TiO2 (0.05 g) | 473 | 5 min | 63.8 | 18.6 | 51 |
| 2b | Glucose (0.1 g) | ZrO2 (0.05 g) | 473 | 5 min | 56.7 | 10.0 | 51 |
| 3c | Glucose (0.1 g) | TiO2–N-773 (0.1 g) | 523 | 5 min | ∼72 | ∼22 | 52 |
| 4c | Glucose (0.1 g) | TiO2–Cl-773 (0.1 g) | 523 | 5 min | ∼79 | ∼28 | 52 |
| 5c | Glucose (0.1 g) | ZrO2–N-773 (0.1 g) | 523 | 5 min | ∼70 | ∼15 | 52 |
| 6c | Glucose (0.1 g) | ZrO2–Cl-773 (0.1 g) | 523 | 5 min | ∼75 | ∼18 | 52 |
| 7c | Glucose (0.1 g) | SO4(1.8)–ZrO2-773 (0.1 g) | 523 | 5 min | ∼62 | ∼22 | 52 |
| 8c | Cellulose (0.1 g) | TiO2–N-773 (0.1 g) | 523 | 5 min | ∼53 | ∼9 | 52 |
| 9c | Cellulose (0.1 g) | TiO2–Cl-773 (0.1 g) | 523 | 5 min | ∼60 | ∼13 | 52 |
| 10c | Cellulose (0.1 g) | ZrO2–N-773 (0.1 g) | 523 | 5 min | ∼38 | ∼6 | 52 |
| 11c | Cellulose (0.1 g) | ZrO2–Cl-773 (0.1 g) | 523 | 5 min | ∼45 | ∼8 | 52 |
| 12c | Cellulose (0.1 g) | SO4(1.8)–ZrO2-773 (0.1 g) | 523 | 5 min | ∼55 | ∼11 | 52 |
| 13d | Cellulose (0.25 g) | Bimodal-HZ-5 (0.5 g) | 463 | 4 h | 67 | 46 | 53 |
| 14e | Cellulose (0.2 g) | Cr[(DS)H2PW12O40]3 (0.06 mmol) | 423 | 2 h | 77.1 | 52.7 | 61 |
In another work, TiO2, ZrO2, and SO4–ZrO2 were used as heterogeneous catalysts for the dehydration of glucose and cellulose to HMF in hot compressed water.52 TiO2 and SO4–ZrO2 were found to be active for hydrolysis and dehydration reactions to produce high yields of HMF with less by-products formation, whereas ZrO2 was highly active for isomerization reaction with significant amount of fructose observed. The authors thought this may be caused by the different acidity–basicity properties of these catalysts, which was similar with the results obtained in Qi et al.'s work.51 It was also observed that the starting salt precursor, the sulfur-doping content (for SO4–ZrO2) and the calcination temperature strongly affected the catalytic activity. For example, catalysts prepared from the chloride-based precursors gained higher reactivity compared with those prepared from nitrate-based precursors. For SO4–ZrO2, SO4–ZrO2 with 1.8% sulfur content presented the highest activity. Meanwhile, the suitable calcination temperature for all catalysts was at 773 K. Under the optimized reaction temperature of 523 K with a reaction time of 5 min, the HMF yields from glucose dehydration were about 22%, 28%, 15%, 18%, and 22% (Table 1, entries 3–7) over TiO2–N (from the precipitation of TiO(NO3)2 calcined at 773 K), TiO2–Cl (from the precipitation of TiCl4 calcined at 773 K), ZrO2–N (from the precipitation of ZrO(NO3)2 calcined at 773 K), ZrO2–Cl (from the precipitation of ZrCl4 calcined at 773 K), and SO4(1.8)–ZrO2-773 (1.8% sulfur content calcined at 773 K), respectively. However, the yields of HMF from cellulose were only 9%, 13%, 6%, 8%, and 11%, respectively, in the presence the above corresponding catalysts (Table 1, entries 8–12).
2.2. Zeolites for dehydration of cellulose in water
Zeolites, a unique class of crystalline aluminosilicates with well-defined channels or cavities, possess both Brønsted acid sites and Lewis acid sites.53,54 Owing to tunable acidities, superior thermostabilities and excellent shape-selectivities, zeolites have been successfully used for the dehydration of cellulose in water.Bimodal-HZ-5 zeolite was prepared by post-synthesis modification of H-ZSM-5 with desilication,55 and it was found that the synthesized bimodal-HZ-5 zeolite could be used as an effective heterogeneous catalyst for dehydration of cellulose to HMF in water. Under the optimized reaction conditions (463 K and 4 h), the maximum cellulose conversion of 67% and HMF yield of 46% were obtained over the obtained bimodal-HZ-5 (Table 1, entry 13). Additionally, the authors found that the synthesized bimodal-HZ-5 zeolite was stable for four catalytic cycles.
2.3. SO42−/Ti-MCM-41 for dehydration of cellulose in water
MCM-41 is a mesoporous material with highly ordered mesoporosity, large surface area, and high hydrothermal stability.56 The incorporation of heteroatoms (Al and Ti) or modified with sulfuric acid can increase the acidity of MCM-41.57 In this aspect, Jiang et al. designed SO42−/Ti-MCM-41 for direct dehydration of cellulose to HMF in hot water (463–503 K). Based on the typical kinetic experiments, a comprehensive mechanism was proposed to describe the heterogeneous dehydration of cellulose to HMF over SO42−/Ti-MCM-41 (Fig. 3). Furthermore, based on the mechanism and the mass balance law, they also proposed a corresponding kinetic model. | ||
| Fig. 3 Comprehensive mechanism for conversion of cellulose to HMF using SO42−/Ti-MCM-41 as a heterogeneous catalyst. Reproduced from ref. 57 with permission from the Royal Society of Chemistry. | ||
2.4. Heteropoly acid as heterogeneous catalysts for dehydration of cellulose in water
Heteropoly acids are made up of a particular combination of hydrogen cations and polyoxometalate anions, and have been used as efficient acidic catalysts for various organic reactions.58–60 For direct conversion of cellulose to HMF in water, a Brønsted–Lewis-surfactant-combined heteropolyacid (HPA) Cr[(DS)H2PW12O40]3 (DS represented OSO3C12H25 dodecyl sulfate) was synthesized and used as a heterogeneous catalyst for the reaction.61 The obtained Cr[(DS)H2PW12O40]3 owning double Brønsted and Lewis acidities, could catalyze the conversion of cellulose into sugar and dehydration of sugar into HMF in a tandem reaction. Meanwhile, this micellar HPA catalyst provided a hydrophobic environment for protecting HMF from further decomposition and decreasing the by-products, which was beneficial for the formation of HMF. Under optimized reaction conditions (423 K and 2 h), a HMF yield of 52.7% could be achieved with a cellulose conversion of 77.1% (Table 1, entry 14).3. Heterogeneous dehydration of glucose and cellulose in organic solvents
For HMF production from heterogeneous dehydration of glucose and cellulose, the effective organic solvents are generally dipolar aprotic solvents, such as dimethylsulfoxide (DMSO), N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMA), N-methyl pyrrolidone (NMP), and acetonitrile, etc. Due to these organic solvents can inhibit the side reactions to some extent, the yields of HMF in them are generally better than those achieved using water as the solvent.3.1. Metal oxides for dehydration of glucose and cellulose in organic solvents
As mentioned in the Section 2.1., metal oxides are an important type of heterogeneous catalysts for HMF production from glucose and cellulose in water. Similarly, they are also efficient heterogeneous catalysts in organic solvents for the dehydration reaction.SO42−/ZrO2 and SO42−/ZrO2–Al2O3 was prepared by impregnation of Zr(OH)4 and Zr(OH)4–Al(OH)3 with ethylene dichloride solution of chlorosulfonic acid and subsequent calcination at 823 K for 4 h.62 The obtained SO42−/ZrO2 and SO42−/ZrO2–Al2O3 could be used as solid acidic catalysts for glucose dehydration to HMF in DMSO. It was found that these catalysts had both Brønsted and Lewis acid sites and the former was stronger. Meanwhile, the base sites on the catalysts increased with the increasing amount of Al, which promoted the glucose-to-fructose isomerization, and thus was beneficial for the dehydration of glucose (Table 2, entries 1–6). Therefore, SO42−/ZrO2–Al2O3 was more active than SO42−/ZrO2. In particular, over SO42−/ZrO2–Al2O3 with Zr–Al mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a HMF yield of about 47.6% was obtained at 403 K with a reaction time of 5 h under N2 protection. In addition, the catalyst could be recovered and reused, and a stable HMF yield of about 35% could be obtained.
1, a HMF yield of about 47.6% was obtained at 403 K with a reaction time of 5 h under N2 protection. In addition, the catalyst could be recovered and reused, and a stable HMF yield of about 35% could be obtained.
| Entry | Substrate | Solvent | Catalyst | Ta (K) | ta | Ca (%) | Ya (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
a T = temperature; t = time; C = conversion; Y = HMF yield.b The concentration of glucose in DMSO was 7.6 wt%, and the amount of catalyst was 18 mg with a glucose/catalyst weight ratio of 5/1.c The mole ratio of Zr and Al was 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.d The mole ratio of Zr and Al was 7 1.d The mole ratio of Zr and Al was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.e The mole ratio of Zr and Al was 1 3.e The mole ratio of Zr and Al was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.f The mole ratio of Zr and Al was 3 1.f The mole ratio of Zr and Al was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.g The mole ratio of Zr and Al was 1 7.g The mole ratio of Zr and Al was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.h The mole ratio of Al and B was 5 9.h The mole ratio of Al and B was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.i β-Cyclodextrin-SO3H was prepared by hydrothermal carbonization of the mixture of β-cyclodextrin and p-toluenesulfonic acid (p-TSA).j Volume ratio of H2O–DMSO and H2O–DMA = 1 5.i β-Cyclodextrin-SO3H was prepared by hydrothermal carbonization of the mixture of β-cyclodextrin and p-toluenesulfonic acid (p-TSA).j Volume ratio of H2O–DMSO and H2O–DMA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.k Poly(VMPS)-PW = poly(1-vinyl-3-propane sulfonate imidazolium)-H3PW12O40. 3.k Poly(VMPS)-PW = poly(1-vinyl-3-propane sulfonate imidazolium)-H3PW12O40. |
||||||||
| 1b | Glucose | DMSO | SO42−/ZrO2 | 403 | 4 h | 95.2 | 19.2 | 62 |
| 2b | Glucose | DMSO | SO42−/ZrO2–Al2O3c | 403 | 4 h | 97.3 | 27.3 | 62 |
| 3b | Glucose | DMSO | SO42−/ZrO2–Al2O3d | 403 | 4 h | 98.1 | 41.5 | 62 |
| 4b | Glucose | DMSO | SO42−/ZrO2–Al2O3e | 403 | 4 h | 97.2 | 47.6 | 62 |
| 5b | Glucose | DMSO | SO42−/ZrO2–Al2O3f | 403 | 4 h | 97.8 | 43.1 | 62 |
| 6b | Glucose | DMSO | SO42−/ZrO2–Al2O3g | 403 | 4 h | 95.2 | 37.0 | 62 |
| 7 | Glucose (50 mg) | DMSO (1 g) | Al2O3–B2O3 (20 mg)h | 413 | 2 h | 94.8 | 41.4 | 63 |
| 8 | Glucose (0.1 g) | DMSO (10 ml) | β-Cyclodextrin-SO3H (0.1 g)i | 453 | 5 h | — | 47 | 66 |
| 9 | Glucose (0.1 g) | DMF (10 ml) | β-Cyclodextrin-SO3H (0.1 g) | 453 | 5 h | — | 37 | 66 |
| 10 | Glucose (0.1 g) | NMP (10 ml) | β-Cyclodextrin-SO3H (0.1 g) | 453 | 5 h | — | 24 | 66 |
| 11 | Glucose (0.1 g) | DMA (10 ml) | β-Cyclodextrin-SO3H (0.1 g) | 453 | 5 h | — | 29 | 66 |
| 12 | Glucose (0.1 g) | Sulpholane (10 ml) | β-Cyclodextrin-SO3H (0.1 g) | 453 | 5 h | — | 28 | 66 |
| 13 | Glucose (0.1 g) | [Bmim]Cl (10 ml) | β-Cyclodextrin-SO3H (0.1 g) | 453 | 5 h | — | 32 | 66 |
| 14 | Glucose (0.1 g) | DMSO (2 ml) | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 4 h | 99 | 43.5 | 70 |
| 15 | Glucose (0.1 g) | DMF (2 ml) | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 4 h | 97 | 12.5 | 70 |
| 16 | Glucose (0.1 g) | DMA (2 ml) | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 4 h | 89.3 | 15.7 | 70 |
| 17 | Glucose (0.1 g) | NMP (2 ml) | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 4 h | 98 | 28.9 | 70 |
| 18 | Glucose (0.1 g) | [Bmim]Cl (2 ml) | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 4 h | 95 | 14.2 | 70 |
| 19 | Glucose (0.1 g) | H2O–DMSO (2 ml)j | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 4 h | 80.5 | 21.4 | 70 |
| 20 | Glucose (0.1 g) | H2O–DMA (2 ml)j | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 4 h | 81.1 | 16.5 | 70 |
| 21 | Cellulose (0.1 g) | DMSO (2 ml) | Cr(salen)-IM-HSO4-MCM-41 (50 mg) | 413 | 5 h | 57.8 | 7.8 | 70 |
| 22 | Glucose (0.1 g) | DMF (3 ml) | Amberlyst-15 (0.1 g) + hydrotalcite (0.2 g) | 373 | 3 h | 72 | 41 | 71 |
| 23 | Glucose (0.1 g) | DMA (3 ml) | Amberlyst-15 (0.1 g) + hydrotalcite (0.2 g) | 373 | 3 h | 97 | 14 | 71 |
| 24 | Glucose (0.1 g) | DMSO (3 ml) | Amberlyst-15 (0.1 g) + hydrotalcite (0.2 g) | 373 | 3 h | 94 | 12 | 71 |
| 25 | Glucose (0.1 g) | Acetonitrile (3 ml) | Amberlyst-15 (0.1 g) + hydrotalcite (0.2 g) | 373 | 3 h | 88 | 10 | 71 |
| 26 | Glucose (50 mg) | DMSO (0.5 ml) | Poly(VMPS)-PW (30 mg)k | 403 | 3 h | — | 26 | 72 |
In another work, the conversion of glucose to HMF could be catalyzed by nano-sized mesoporous Al2O3–B2O3 in DMSO.63 The molar ratio of Al and B affected the activity of these catalysts due to the change of the amount of Lewis acids, amorphous state, and pore diameter under different Al–B ratios. The catalyst with Al–B ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 showed the best performance, and the highest HMF yield of 41.4% could be obtained with a glucose conversion of 94.8% at 413 K for 2 h (Table 2, entry 7). In addition, the activity in DMSO was found to be better than those conducted in DMA, DMF, ionic liquid (IL), and mixed system consisting of IL and biphasic system.
5 showed the best performance, and the highest HMF yield of 41.4% could be obtained with a glucose conversion of 94.8% at 413 K for 2 h (Table 2, entry 7). In addition, the activity in DMSO was found to be better than those conducted in DMA, DMF, ionic liquid (IL), and mixed system consisting of IL and biphasic system.
3.2. Carbonaceous solid acid for dehydration of glucose in organic solvents
Carbonaceous acids, an amorphous carbon material consisting of small polycyclicaromatic carbon sheets with a high density of sulfonic acid (SO3H) sites, are an attractive class of heterogeneous acidic catalysts due to their low cost, metal-free composition, and high stability and reusability.64,65 Carbonaceous solid acids show very promising catalytic activity for many acid-catalyzed reactions. Inspired by the reported results, a β-cyclodextrin-SO3H carbonaceous solid acidic catalyst was prepared by hydrothermal carbonization of the mixture of β-cyclodextrin and p-toluenesulfonic acid (p-TSA) at 453 K for 24 h under N2 and further for 24 h keeping open to air (Fig. 4).66 The synthesized carbonaceous solid acid, having –SO3H, –COOH and –OH groups, could be used as efficient heterogeneous acidic catalyst for dehydration of glucose to HMF in DMSO. The HMF yield in DMSO was 47% from glucose at 453 K with a reaction time of 5 h, which was higher than the results obtained in other solvents (Table 2, entries 8–13), such as DMF, DMA, NMP, sulpholane, and 1-butyl-3-methylimidazolium chloride ([Bmim]Cl). | ||
| Fig. 4 Preparation of β-cyclodextrin-SO3H carbonaceous catalyst and its application in HMF synthesis. Reproduced from ref. 66 with permission from Elsevier. | ||
3.3. Functionalized silicas for dehydration of glucose and cellulose in organic solvents
Silicas are a type of inorganic materials with diverse properties. Generally, silicas can be divided into amorphous silicas (SiO2) and crystalline silicas (e.g. SBA-15 and MCM-41), which are not proper catalysts for dehydration of glucose due to their very weak even negligible acidities.67 However, silicas can be functionalized by the introduction of acidic groups and other active groups to form efficient solid acidic catalysts.68,69 In this respect, Wang et al. prepared a series of functional silicas by immobilizing chromium(III) Schiff base complexes and acidic ionic liquids onto the surface of MCM-41 (Fig. 5) for conversion of glucose and cellulose to HMF in DMSO.70 The highest HMF yield of 43.5% could be achieved from glucose using Cr(salen)-IM-HSO4-MCM-41 as the catalyst in DMSO at 413 K for 4 h, which was higher than the results obtained in DMF, DMA, NMP, [Bmim]Cl, and the mixture of H2O–DMSO, H2O–DMA, and [Bmim]Cl–DMSO (Table 2, entries 14–20). Based on the results, the authors proposed a possible reaction mechanism for glucose conversion to HMF (Fig. 6): firstly, glucose was isomerized to fructose with the catalytic effect of Cr(III); secondly, the generated fructose was dehydrated to HMF by the H+. The catalyst could be successfully recycled for five experiments with only a minor decrease in catalytic activity. Additionally, this prepared catalyst could catalyze the dehydration of cellulose to HMF, but unfortunately, the yield was only 7.8% because the hydrolysis of cellulose to glucose was difficult in DMSO (Table 2, entry 21).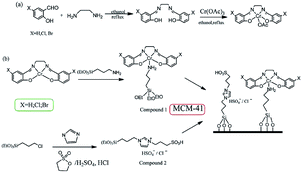 | ||
| Fig. 5 (a) Synthetic route to the chromium(III) complexes; (b) synthetic route to the Cr-mesoporous silica supported catalyst. Reproduced from ref. 70 with permission from the Royal Society of Chemistry. | ||
 | ||
| Fig. 6 Plausible reaction mechanism for the conversion of glucose into HMF on the Cr(salen)-IM-HSO4-MCM-41 catalyst. Reproduced from ref. 70 with permission from the Royal Society of Chemistry. | ||
3.4. Functional polymers for dehydration of glucose in organic solvents
Polymers with various functional groups have huge potential in catalysis due to their large surface area, high stability, and tunable properties by changing the monomers. When modified with acidic groups (e.g. –SO3H, –COOH), polymers can be used as heterogeneous acidic catalysts for dehydration of glucose in organic solvents.The dehydration of glucose to HMF could be catalyzed by a combination of solid acid (Amberlyst-15, a resin) and base (hydrotalcite) in DMF.71 In the catalytic system, hydrotalcite was used as the basic catalyst for isomerization of glucose to fructose, and Amberlyst-15 was used as the acidic catalyst for dehydration of fructose to HMF (Fig. 7). A HMF yield of 41% could be obtained in this system with a glucose conversion of 72%, which was higher than the results achieved in other solvents, including DMA, DMSO, acetonitrile, DMF–H2O, and acetonitrile–H2O (Table 2, entries 22–25). In addition, the authors found that the catalysts were recyclable.
 | ||
| Fig. 7 Dehydration of glucose to HMF catalyzed by Amberlyst-15 and hydrotalcite. Reproduced from ref. 71 with permission from Elsevier. | ||
Subsequently, poly(1-vinyl-3-propane sulfonate imidazolium)-H3PW12O40 (poly(VMPS)-PW) was synthesized and used as a heterogeneous Brønsted-acidic catalyst for dehydration of glucose (Fig. 8).72 Under optimized conditions (423 K and 3 h), a HMF yield of 26% was obtained from glucose in DMSO (Table 2, entry 26). The relatively low yield from glucose may be attributable to the catalytic process primarily proceeding via 3-deoxy-D-erythro-hex-2-ulose (3-deoxyglucosone, Fig. 8) and also because of the lack of active sites in the solid catalyst for the isomerization of glucose to fructose. In addition, poly(VMPS)-PW could be reused five times without significant loss of activity.
 | ||
| Fig. 8 Selective conversion of glucose to HMF catalyzed by poly(VMPS)-PW. Reproduced from ref. 72 with permission from John Wiley and Sons. | ||
4. Heterogeneous dehydration of glucose and cellulose in ionic liquids
Ionic liquids (ILs) have attracted enormous attention in recent years, owing to their unusual properties. More importantly, the properties of ILs related with hydrophobicity, polarity, and solvent power can be tuned by appropriate combination or modification of the cations and anions.73–76 Due to the unique properties, ILs have been applied as efficient solvents in many fields. In recent years, a lot of works for conversion of glucose and cellulose to HMF in ILs have been reported inspired by Zhao et al.'s work.23 Herein, we only highlight the dehydration of glucose and cellulose into HMF in ILs over various heterogeneous catalysts.4.1. Cr-based solid catalysts for dehydration of glucose and cellulose in ILs
It has been reported that Cr-based Lewis acids show excellent performance for dehydration of glucose and cellulose to HMF in ILs.23,24 However, the separation and reusability of the catalysts are difficult. Therefore, design of heterogeneous Cr-based catalysts is very attractive for dehydration of glucose and cellulose in ILs. In this aspect, several works have been reported recently.Hydroxyapatite supported chromium chloride (Cr-HAP) was prepared as heterogeneous catalyst for dehydration of glucose in IL [Bmim]Cl with the aid of microwave irradiation.77 A HMF yield of 40.2% could be obtained with a glucose conversion of 77.9% in 2.5 min (Table 3, entry 1). 13C NMR spectra indicated that Cr-HAP could promote glucose from the form of α-pyranose to β-pyranose in [Bmim]Cl as described by Zhao et al.,23 and the formed β-pyranose could subsequently form enolate anion complex, which would be converted to fructose by a isomerization step. In addition, Cr-HAP could be reused at least five times and the catalytic activity was remained.
| Entry | Substrate | Solvent | Catalyst | Ta (K) | ta | Ca (%) | Ya (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a T = temperature; t = time; C = conversion; Y = HMF yield.b Cr-HAP = hydroxyapatite supported chromium chloride.c MI = microwave irradiation at 400 W.d K-10 clay-Cr = chromium-exchanged montmorillonite K-10 clay.e ATP = attapulgite, HNTs = halloysite nanotubes.f Zr-P-Cr = Cr-incorporated mesoporous zirconium phosphate.g D001-cc, NKC-9, and D072 were strong-acidic cation-exchange resins.h D152 was macroporous weak-acidic cation-exchange resin.i ATP = attapulgite, HNTs = halloysite nanotubes, PSt = polystyrene, and DVB = polydivinylbenzene.j Catalyst-110° = Brønsted acidic polymer nanotubes with hydrophobic surface wettability, and catalyst-10° = Brønsted acidic polymer nanotubes with hydrophilic surface wettability. | ||||||||
| 1 | Glucose (0.1 g) | [Bmim]Cl (2 g) | Cr-HAP (100 mg)b | MIc | 2.5 min | 77.9 | 40.2 | 77 |
| 2 | Glucose (180 mg) | [Bmim]Cl (4 g) | K-10 clay-Cr (160 mg)d | 393 | 2 h | — | 56.3 | 78 |
| 3 | Cellulose (180 mg) | [Bmim]Cl (4 g) | K-10 clay-Cr (160 mg)d | 423 | 6 h | — | 48.7 | 78 |
| 4 | Cellulose (0.1 g) | [Emim]Cl (2 g) | ATP-SO3H-Cr(III) (0.1 g)e | 393 | 2 h | — | 31.2 | 79 |
| 5 | Cellulose (0.1 g) | [Emim]Cl (2 g) | HNTs-SO3H-Cr(III) (0.1 g)e | 393 | 2 h | — | 41.22 | 79 |
| 6 | Glucose (180 mg) | [Bmim]Cl (4 g) | Zr-P-Cr (100 mg)f | 393 | 12 h | 94.7 | 43.2 | 80 |
| 7 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Cr3+-D001-cc resin (0.1 g)g | 383 | 30 min | — | 61.3 | 81 |
| 8 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Cr2+-D001-cc resin (0.1 g) | 383 | 30 min | — | 60.7 | 81 |
| 9 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Cu2+-D001-cc resin (0.1 g) | 383 | 30 min | — | 15.3 | 81 |
| 10 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Mg2+-D001-cc resin (0.1 g) | 383 | 30 min | — | 6.2 | 81 |
| 11 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Fe3+-D001-cc resin (0.1 g) | 383 | 30 min | — | 14.4 | 81 |
| 12 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Al3+-D001-cc resin (0.1 g) | 383 | 30 min | — | 20.2 | 81 |
| 13 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Mo5+-D001-cc resin (0.1 g) | 383 | 30 min | — | 5.4 | 81 |
| 14 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Cr3+-NKC-9 resin (0.1 g)g | 383 | 30 min | — | 48.8 | 81 |
| 15 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Cr3+-D072 resin (0.1 g)g | 383 | 30 min | — | 50.3 | 81 |
| 16 | Glucose (0.1 g) | [Bmim]Cl (1.5 g) | Cr3+-D152 resin (0.1 g)h | 383 | 30 min | — | 6.9 | 81 |
| 17 | Cellulose (0.1 g) | [Emim]Cl (2 g) | HNTs-PSt-PDVB-SO3H(I) (0.1 g)i | 393 | 2 h | — | 28.22 | 82 |
| 18 | Cellulose (0.1 g) | [Emim]Cl (2 g) | HNTs-PSt-PDVB-SO3H(II) (0.05 g)i | 393 | 2 h | — | 32.86 | 82 |
| 19 | Cellulose (0.1 g) | [Emim]Cl (2 g) | Catalyst-110° (30 mg)j | 393 | 30 min | — | 34.6 | 83 |
| 20 | Cellulose (0.1 g) | [Emim]Cl (2 g) | Catalyst-10° (30 mg)j | 393 | 30 min | — | 37.1 | 83 |
| 21 | Cellulose (0.1 g) | [Emim]Cl (2 g) | SPHs@MSNs-SO3H-NH2 (40 mg) | 393 | 30 min | — | 44.5 | 84 |
| 22 | Cellulose (0.1 g) | [Emim]Cl (2 g) | PDVB-SS-0.2-SO3H (40 mg) | 393 | 2 h | — | 29.2 | 85 |
| 23 | Cellulose (0.1 g) | [Emim]Cl (2 g) | PDVB-SS-0-SO3H (40 mg) | 393 | 2 h | — | 12.9 | 85 |
| 24 | Cellulose (0.1 g) | [Emim]Cl (2 g) | PDVB-SS-0.6-SO3H (40 mg) | 393 | 2 h | — | 15.5 | 85 |
| 25 | Glucose (0.1 g) | [Bmim]Cl (1 g) | Hβ-Zeolite (Si/Al = 25) (40 mg) | 413 | 50 min | 80.6 | 50.3 | 86 |
| 26 | Cellulose (0.1 g) | [Bmim]Cl (1 g) | Hβ-Zeolite (Si/Al = 25) (40 mg) | 423 | 50 min | — | 45.4 | 86 |
| 27 | Glucose (0.1 g) | [Bmim]Br (1 g) | Sn-MCM-41 (0.1 g) | 383 | 4 h | 99 | 70 | 87 |
| 28 | Glucose (0.1 g) | [Bmim]Cl (1 g) | Cellulose-derived carbonaceous catalyst (40 mg) | 433 | 15 min | — | 46.4 | 88 |
Chromium-exchanged montmorillonite K-10 clay was synthesized and used as the heterogeneous catalyst for dehydration of glucose in [Bmim]Cl by Fang et al.78 A HMF yield of 56.3% was obtained from glucose at 393 K with a reaction time of 2 h (Table 3, entry 2). More delighting, the obtained catalyst could also catalyze the dehydration of cellulose efficiently in [Bmim]Cl, and a satisfied HMF yield of 48.7% could be obtained at 423 K with a reaction time of 6 h (Table 3, entry 3). In addition, the catalyst could be effectively recycled six times without significant loss of activity.
Two acid-chromic chloride bi-functionalized catalysts (i.e., ATP-SO3H-Cr(III) and HNTs-SO3H-Cr(III)) were prepared by grafting –SO3H and Cr(III) onto the surface of treated attapulgite (ATP) and halloysite nanotubes (HNTs).79 These two bi-functional catalysts could catalyze the direct dehydration of cellulose to HMF in 1-ethyl-3-methyl-imidazolium chloride ([Emim]Cl). Under optimized conditions (393 K and 2 h), the yield of HMF up to 31.20% and 41.22% was obtained over ATP-SO3H-Cr(III) and HNTs-SO3H-Cr(III), respectively (Table 3, entries 4 and 5). In addition, the prepared catalysts could be very easily recycled at least three times without significant loss of activity.
Furthermore, Cr-incorporated mesoporous zirconium phosphate was used as the heterogeneous catalyst for effective conversion of glucose to HMF in [Bmim]Cl.80 A moderate HMF yield of 43.2% could be achieved under the best reaction conditions (393 K and 12 h), but the conversion of glucose was high to 94.7% (Table 3, entry 6). This may be caused by the inefficient isomerization of glucose into fructose. More importantly, the Cr-incorporated mesoporous zirconium phosphate was stable during the reaction process, and could be reused for several times without the significant loss of its catalytic activity.
Recently, Cr3+-modified ion exchange resin (Cr3+-D001-cc resin) was prepared by Liu et al. through a simple ion exchange between D001-cc cation-exchange resin and CrCl3 (Fig. 9).81 This Cr3+-modified ion exchange resin showed better performance for dehydration of glucose than other ion-exchange catalysts (e.g. Cu2+, Mo5+, Fe3+, Mg2+, Al3+), and a HMF yield of 61.3% was achieved in [Bmim]Cl at 383 K with a reaction time of 30 min (Table 3, entries 7–16). The higher activity of Cr3+-D001-cc resin was due to that Cr3+ could promote the isomerization of glucose to fructose, whereas other metal ions could only improve the mutarotation of α-glucopyranose anomer to β-glucopyranose anomer. The Cr3+-D001-cc resin could also catalyze dehydration of cellulose under the same reaction conditions with glucose, but the HMF yield was only 2.4%, which was caused by the difficulty of the hydrolysis of cellulose under the waterless environment and lower temperature employed in the experiment. Recyclability experiments indicated that the catalytic activity of the Cr3+-D001-cc/[Bmim]Cl system showed an obvious decrease after the sixth run, which was caused by the damage of resin and adsorption of the by-products in the reaction process.
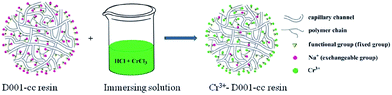 | ||
| Fig. 9 Diagrammatic sketch of the preparation of Cr3+-D001-cc resin. Reproduced from ref. 81 with permission from the Royal Society of Chemistry. | ||
4.2. Polymer-based heterogeneous catalysts for dehydration of cellulose in ILs
As mentioned in Section 3.4., functional polymers are attractive heterogeneous catalysts for dehydration of glucose and cellulose. In terms of dehydration of cellulose in ILs, a series of works have been conducted by employing functional polymers as the heterogeneous catalysts.Based on halloysite nanotubes (HNTs), Zhang et al. synthesized two composites, i.e. HNTs-polystyrene (PSt)-polydivinylbenzene (DVB)(I) and HNTs-PSt-PDVB(II), through precipitation polymerization and Pickering emulsion polymerization, respectively.82 After sulfonation by 98% H2SO4, two polymeric solid acidic catalysts, i.e. HNTs-PSt-PDVB-SO3H(I) and HNTs-PSt-PDVB-SO3H(II), were successfully prepared for direct conversion of cellulose to HMF in [Emim]Cl. HNTs-PSt-PDVB-SO3H(II) with more very strong acidic sites showed better catalytic performance than HNTs-PSt-PDVB-SO3H(I), indicating the key role of strong acidic sites in cellulose dehydration to HMF. Under the optimized conditions, the HMF yields could reach 28.52% for HNTs-PSt-PDVB-SO3H(I) and 32.86% for HNTs-PSt-PDVB-SO3H(II) (Table 3, entries 17 and 18). In addition, the two as-prepared catalysts could be very easily recycled. In a following work, they prepared two Brønsted acidic polymer nanotubes by chemical conjugating grafting –SO3H groups onto the surface of polydivinylbenzene (PDVB) nanotubes, which were derived from cationic polymerization of divinylbenzene.83 By adjusting the grafting amounts of hydrophilic –SO3H groups, the obtained catalysts (i.e., catalyst-110° and catalyst-10°) could possess hydrophobic and hydrophilic surface wettability. For cellulose dehydration to HMF, the catalyst-110° (HMF yield was about 34.6%) showed comparable catalytic activity to catalyst-10° (HMF yield was about 37.1%) in [EMIM]Cl although the acid strength of catalyst-10° was higher than that of catalyst-110°, indicating that the hydrophobic nature of catalyst-110° was beneficial for decreasing side-reaction of HMF (Table 3, entries 19 and 20). Furthermore, both catalysts can be easily recovered and reused for at least four times without significant loss of their catalytic activities. Very recently, they prepared a hierarchically macro-/mesoporous SPHs@MSNs-SO3H-NH2 catalyst containing both acidic and basic sites through the chemical combination of acid–base bifunctionalized mesoporous silica nanoparticles (MSNs-SO3H-NH2) with macroporous polymer foam poly(HIPE) (PH) matrix PHs derived from water-in-oil high internal phase emulsion templating, followed by a sulfonation process.84 The newly synthesized solid catalyst can be used for highly efficient synthesis of HMF from cellulose in [Emim]Cl. In the catalyst, the basic sites enhanced the isomerization of glucose to fructose, while the acidic sites catalyzed the hydrolysis of cellulose to glucose and the dehydration of fructose to HMF (Fig. 10). Therefore, the prepared catalyst with both acidic and basic sites showed good performance for dehydration of cellulose, and a HMF yield of 44.5% could be obtained. Additionally, the obtained catalyst could catalyze the dehydration of glucose to HMF in DMSO/H2O system with a HMF yield of 66.9% resulted from the hierarchically macro-/mesoporous structure and acid–base bifunctions (Table 3, entry 21).
 | ||
| Fig. 10 Catalyst behavior of SPHs@MSNs-SO3H-NH2 catalyst for conversion of cellulose to HMF. Reproduced from ref. 84 with permission from John Wiley and Sons. | ||
Several macroporous polymerized solid acid (PDVB-SS-X-SO3H, X = 0, 0.2, 0.6) were prepared by polymerizing divinyl benzene (DVB) and sodium p-styrenesulfonate (SS) in water-in-oil Pickering high internal phase emulsions, followed by a sulfonation process.85 These obtained solid catalysts was used for conversion of cellulose to HMF in [Emim]Cl (Fig. 11), and was found that a maximum yield of 29.6% for PDVB-SS-0.2-SO3H, 12.9% for PDVB-SS-0-SO3H and 15.5% for PDVB-SS-0.6-SO3H under the same condition within 2.0 h at 393 K (Table 3, entries 22–24). DVB-SS-0.2-SO3H showed the best performance, which was resulted from two aspects: first, the smaller pore sizes helped in increasing contact frequency between reaction substrates and catalytic acid sites; second, moderate amounts of strong acid was more conducive for the improvement of the catalytic effect.
 | ||
| Fig. 11 PDVB-SS-X-SO3H catalyzed conversion of cellulose into HMF in [Emim]Cl. Reproduced from ref. 85 with permission from the Royal Society of Chemistry. | ||
4.3. Other heterogeneous catalysts for dehydration of glucose and cellulose in ILs
Various zeolites (including HY-zeolite, H-mordenite, HZSM-5, and Hβ-zeolite) was found to be efficient catalyst for the dehydration of glucose to HMF in [Bmim]Cl.86 Hβ-Zeolite with a unique BEA structure and a moderate Si/Al ratio of 25 possessed the highest catalytic activity, which resulted in a HMF yield of 50.3% with the glucose conversion of 80.6% at a reaction temperature of 423 K for only 50 min (Table 3, entry 25). Through the study of the mechanism (Fig. 12), the authors thought that there were four aspects for the good catalytic activity of Hβ-zeolite/[Bmim]Cl system. Firstly, glucose was completely dissolved in [Bmim]Cl, which made the catalyst more accessible to glucose. Secondly, Cl− in [Bmim]Cl was very helpful to Lewis acid sites of Hβ-zeolite for the isomerization of glucose to fructose. Thirdly, the ion-exchange of [Bmim]Cl with Brønsted acid sites of Hβ-zeolite promoted the release of H+, which could readily catalyze dehydration of fructose to HMF. Finally, [Bmim]+ in [Bmim]Cl could stabile HMF and then avoid the further decomposition of HMF. In addition, HMF yield was gradually decreased to 35.6% when Hβ-zeolite (Si/Al = 25) was continuously used three times, which was caused by deposition of humins on Hβ-zeolite. The catalytic activity could be easily regenerated by a simple calcination at 823 K. Furthermore, Hβ-zeolite/[Bmim]Cl catalytic system was also effective for dehydration of cellulose with a HMF yield of 45.4% obtained at 423 K with a reaction time of 50 min (Table 3, entry 26).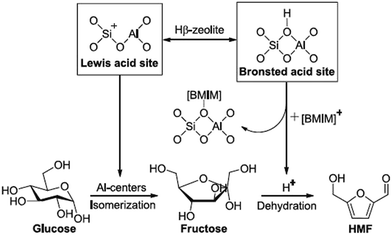 | ||
| Fig. 12 The plausible mechanism for the conversion of glucose into HMF in the presence of Hβ-zeolite and [Bmim]Cl. Reproduced from ref. 86 with permission from Elsevier. | ||
Furthermore, a tin-containing silica molecular sieve (Sn-MCM-41) was synthesized as the bifunctional heterogeneous catalyst for dehydration of glucose to HMF in 1-butyl-3-methylimidazolium bromide ([Bmim]Br).87 The HMF yield could reach up to 70% at 110 °C with a reaction time of 4 h (Table 3, entry 27). After the reaction, the heterogeneous Sn-MCM-41 could be easily recovered and reused without a significant loss in activity.
Cellulose-derived carbonaceous catalyst containing –SO3H, –COOH and phenolic –OH groups was developed through incomplete carbonization and subsequent sulfonation.88 The obtained carbonaceous catalyst could be used as heterogeneous catalyst for conversion of glucose to HMF in [Bmim]Cl, and a HMF yield of 46.4% could be achieved at 433 K for only 15 min (Table 3, entry 28). The authors thought that the high activity of the synthesized carbonaceous catalyst was attributed to the synergic effect of –SO3H, –COOH and phenolic –OH groups: –COOH and phenolic –OH groups had a strong affinity to glucose through the formation of hydrogen bonds between them and –OH groups of glucose, which was beneficial for conversion of glucose to HMF catalyzed by –SO3H groups. Meanwhile, the catalytic system was also effective for direct conversion of cellulose to HMF with a yield of 40.5% under the same reaction conditions as glucose. In addition, [Bmim]Cl was also found to be very important for the reaction due to the following three reasons: (i) [Bmim]Cl could completely dissolve glucose and cellulose to generate homogeneous solutions, which made the catalyst more accessible to glucose and cellulose; (ii) Cl− of [Bmim]Cl acting as both a base and a nucleophile could promote the isomerization of glucose to fructose and the dehydration of fructose to HMF; (iii) [Bmim]+ of [Bmim]Cl could stabilize the generated HMF and avoid its further decomposition.
5. Heterogeneous dehydration of glucose and cellulose in mixed solvents
The solvent properties (e.g. viscosity, polarity, solvent power) can be regulated by mixing two or more solvents, which can be helpful for the design of efficient solvent systems to improve the activity and selectivity of some chemical reactions. In this content, some works have been reported for dehydration of glucose and cellulose over various heterogeneous catalysts in some mixed solvents.Sn-Mont catalyst was prepared by Wang et al. through the ion-exchange of natural calcium montmorillonite (Ca-Mont) with an aqueous tin tetrachloride,89 and was used as heterogeneous catalyst for glucose dehydration in DMSO–THF mixed solvents. Sn-Mont in DMSO–THF mixed solvents provided higher HMF yield than the reaction conducted in pure solvent (including THF, water, DMSO, DMF, DMA, and NMP). The HMF yield could reach up to 53.5% at 433 K for 3 h in DMSO (1.8 ml)–THF (4.2 ml) mixed solvent (Table 4, entry 1). In the catalytic cycle, Sn4+ acted as Lewis acid sites to isomerize glucose to fructose, which was dehydrated to HMF by the Sn–OH as the Brønsted acid sites (Fig. 13). It was also found that Sn-Mont was stable in this system. Additionally, it should point out that Sn-Mont was also effective for dehydration of glucose and cellulose in a THF/H2O–NaCl biphasic system, which was the discussed content in the next section. The HMF yields of 59.3% and 39.1% could be achieved from glucose and cellulose (Table 4, entries 2 and 3), respectively, in THF (5 ml)/H2O (1 ml)–NaCl (0.37 g) biphasic system. Here, NaCl not only increased the partitioning coefficient between THF and water, but also inhibited the generated HMF from further dehydration to levulinic acid.
| Entry | Substrate | Solvent | Catalyst | Ta (K) | ta | Ca (%) | Ya (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
a T = temperature; t = time; C = conversion; Y = HMF yield.b Sn-Mont = Sn4+ ion-exchange of natural calcium montmorillonite.c LCC = lignin-derived carbonaceous catalyst.d PEG-OSO3H = polyethylene glycol (PEG)-bound sulfonic acid and PS-PEG-OSO3H = polystyrene-poly(ethylene glycol) (PS-PEG) resin-supported sulfonic acid.e The mole ratio of [Bmim]Cl and [Bmim][BF4] was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.f Si/Sn = 35 and Si/Al = 100.g Volume ratio of DMSO to 50% glucose solution was 2 1.f Si/Sn = 35 and Si/Al = 100.g Volume ratio of DMSO to 50% glucose solution was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.h Ti/Si = 4/1. 1.h Ti/Si = 4/1. |
||||||||
| 1 | Glucose (5 wt%) | DMSO (1.8 ml)–THF (4.2 ml) | Sn-Mont (0.2 g)b | 433 | 3 h | 98 | 53.5 | 89 |
| 2 | Glucose (5 wt%) | THF (5 ml)/H2O (1 ml)–NaCl (0.37 g) | Sn-Mont (0.2 g)b | 433 | 3 h | — | 59.3 | 89 |
| 3 | Cellulose (5 wt%) | THF (5 ml)/H2O (1 ml)–NaCl (0.37 g) | Sn-Mont (0.2 g)b | 433 | 3 h | — | 39.1 | 89 |
| 4 | Glucose (10 wt%) | DMSO (0.8 g)–[Bmim]Cl (1.2 g) | LCC (0.1 g)c | 433 | 50 min | 99 | 68 | 90 |
| 5 | Glucose (2 mmol) | DMSO (4 ml)/H2O (2 ml)–LiCl (0.3 g) | PEG-OSO3H (0.7 g)d | 393 | 1.5 h | 99 | 78 | 91 |
| 6 | Cellulose (2 mmol) | DMSO (4 ml)/H2O (2 ml)–LiCl (0.3 g) | PEG-OSO3H (0.7 g)d | 393 | 1.5 h | 99 | 52 | 91 |
| 7 | Glucose (2 mmol) | DMSO (4 ml)/H2O (2 ml)–LiCl (0.3 g) | PS-PEG-OSO3H (0.2 g)d | 393 | 1 h | 98 | 86 | 91 |
| 8 | Cellulose (2 mmol) | DMSO (4 ml)/H2O (2 ml)–LiCl (0.3 g) | PS-PEG-OSO3H (0.2 g)d | 393 | 1 h | 96 | 54 | 91 |
| 9 | Glucose (25 mg) | [Bmim]Cl–[Bmim][BF4] (0.5 g)e | Amberlyst 15 (25 mg) | 298 | 2 h | — | 32 | 92 |
| 10 | Glucose (10 wt%) | H2O (2 ml)–DMSO (6 ml) | [Sn,Al]-Beta (0.05 g)f | 433 | 4 h | — | 37.3 | 93 |
| 11 | Glucose (25 g) | H2O (50 g)–DMSOg | ZrO2 + SO42−/TiO2–SiO2 (5 g)h | 413 | 12 h | — | 85 | 94 |
| 12 | Glucose (25 g) | H2O (50 g)–DMSOg | SO42−/TiO2–SiO2 (5 g)h | 413 | 12 h | — | 37 | 94 |
 | ||
| Fig. 13 Plausible reaction mechanism for glucose isomerisation to fructose following dehydration to HMF on Sn-Mont catalyst. Reproduced from ref. 89 with permission from the Royal Society of Chemistry. | ||
Six solid carbonaceous acidic catalysts were prepared by carbonization and sulfonation of raw biomass materials, i.e., glucose, fructose, cellulose, lignin, bamboo and Jatropha hulls.90 These obtained catalysts were used for conversion of glucose to HMF in the mixture of DMSO and [Bmim]Cl. It was discovered that the addition of DMSO in [Bmim]Cl could improve the activity of the dehydration due to the decreased viscosity and the inhibiting effect of DMSO on side-reactions, and the DMSO and [Bmim]Cl weight ratio of 4/6 showed the best performance. Furthermore, the catalyst derived from lignin had the best activity than others because more –SO3H groups in other catalysts were surrounded by the highly cross-linked polycyclic aromatic carbon sheets, which made reactant molecules not access to them easily, and thus leading to a low efficiency. Catalyzed by the lignin-derived carbonaceous catalyst, a HMF yield of 68% could be achieved with a glucose conversion of 99% at 433 K for 50 min (Table 4, entry 4).
Polyethylene glycol (PEG)-bound sulfonic acid (PEG-OSO3H) and polystyrene-poly(ethylene glycol) (PS-PEG) resin-supported sulfonic acid (PS-PEG-OSO3H) were used for dehydration of glucose and cellulose to HMF in the mixture of water and another solvent (n-butyl alcohol, THF, DMA, DMF, DMSO, 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim]PF6), glycerol and PEG-300), with the addition of LiCl.91 It was found that the H2O (2 ml)–DMSO (4 ml) mixture showed the best performance because DMSO could favour the furanoid form and suppress the side reactions. Meanwhile, the addition of LiCl could improve the activity and selectivity of the catalytic system because LiCl promoted isomerization of glucose to fructose, followed by dehydration of fructose to HMF (Fig. 14). Under optimized reaction conditions (393 K, 1.5 h for PEG-OSO3H or 1 h for PS-PEG-OSO3H), the yields of HMF could reach up to 78% and 52% from glucose and cellulose over PEG-OSO3H or 86% and 54% over PS-PEG-OSO3H, respectively (Table 4, entries 5–8).
 | ||
| Fig. 14 Possible reaction mechanism for glucose isomerisation to fructose following dehydration to HMF on PEG-OSO3H catalyst with LiCl. Reproduced from ref. 91 with permission from the Royal Society of Chemistry. | ||
Amberlyst 15 could also be used as heterogeneous catalyst for dehydration of glucose in ionic liquid binary mixtures of [Bmim]Cl with other [Bmim]+ based ILs (Fig. 15, [Bmim][BF4], [Bmim][N(CF3SO2)2], [Bmim][N(CN)2], [Bmim][SbF6] and [Bmim][CF3SO3]).92 It was found that the mixture of [Bmim]Cl and [Bmim][BF4] showed the best performance, and when the mole ratio of [Bmim]Cl and [Bmim][BF4] was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the HMF yield was the highest due to a balance between the favorable effect resulting from a higher concentration of Cl−, which was able to form hydrogen bonds with glucose, and the unfavorable one due to the higher viscosity of the solvent medium. The yield of HMF could reach 32% at 298 K with a reaction time of 2 h (Table 4, entry 9).
1, the HMF yield was the highest due to a balance between the favorable effect resulting from a higher concentration of Cl−, which was able to form hydrogen bonds with glucose, and the unfavorable one due to the higher viscosity of the solvent medium. The yield of HMF could reach 32% at 298 K with a reaction time of 2 h (Table 4, entry 9).
 | ||
| Fig. 15 The structure of the used ILs in D'Anna's work. Reproduced from ref. 92 with permission from Elsevier. | ||
[Sn,Al]-Beta catalysts were prepared by a combination of partial dealumination and isomorphous substitution of Sn into the zeolite framework,93 which were used to catalyze the synthesis of HMF from glucose in the mixture of water and another organic solvents (i.e., DMSO, DMF, DMA, and ethanol). In the catalyst, Sn atoms were necessary for the isomerization of glucose to fructose, and Al was needed for the dehydration of fructose to HMF. Detail examinations indicated that DMSO showed better performance than DMF, DMA, and ethanol because of the effect of DMSO discussed above.91 The highest HMF yield of 37.3% could be obtained in water (2 ml)–DMSO (6 ml) at 433 K with a reaction time of 4 h (Table 4, entry 10) in the presence of [Sn,Al]-Beta having suitable Si/Sn (35) and Si/Al (100) mole ratio. In addition, recyclability experiments indicated that [Sn,Al]-Beta had excellent stability in glucose conversion to HMF.
Very recently, the activity of ZrO2, SO42−/TiO2–SiO2, and their mixture for dehydration of glucose to HMF was compared in mixture of water and DMSO.94 It was discovered that higher mole ratio of Ti and Si was beneficial for the formation of HMF due to more Lewis acid sites existed in SO42−/TiO2–SiO2 with higher Ti amount, and the Ti/Si mole ratio of 4/1 provided the best performance. Furthermore, the mixture of ZrO2 and SO42−/TiO2–SiO2 had higher activity than ZrO2 or SO42−/TiO2–SiO2 alone acting as the catalyst (Table 4, entries 11 and 12). In the catalyst mixture, ZrO2 as basic catalyst mainly played the role to promote the isomerization of glucose to fructose, while SO42−/TiO2–SiO2 as acidic catalyst enhanced dehydration of the obtained fructose to HMF. Under optimal conditions (413 K and 12 h), the HMF yield from glucose could reach more than 85% in water–DMSO system (volume ratio of DMSO to 50% glucose solution was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
6. Heterogeneous dehydration of glucose and cellulose in biphasic solvent systems
In some signal phase systems, the activity for dehydration of glucose and cellulose is not high due to the side-reaction and the decomposition of the generated HMF, especially using water as the reaction media. In order to improve the HMF yield, another solvent can be added as an extractant to in situ separate the generated HMF in the reaction process, which will decrease the side-reactions and the decomposition of HMF, and thus increasing the selectivity of HMF ultimately.95 In this content, several organic solvent (e.g. tetrahydrofuran (THF), methyl-iso-butyl ketone (MIBK), n-butanol (n-BuOH)) have been used as efficient extractants for the dehydration, and some good results have also been reported with various heterogeneous catalysts.6.1. Metal oxides for dehydration of glucose and cellulose in biphasic solvent systems
Mesoporous tantalum oxide was prepared through acid hydrolysis of tantalum penta-ethoxide in the presence of a triblock co-polymer Pluronic L-121 and subsequent calcination at 823 K for 6 h.96 The obtained tantalum oxide was active as solid acid catalyst for dehydration of glucose to HMF in water (1.5 g)–MIBK (3.5 ml) system. It was discovered that this catalytic process was selective to HMF. Meanwhile, fructose was also detected from the glucose isomerization due to the Lewis acid sites on the catalysts. A HMF yield of 23% and a glucose conversion of 69% were achieved at 448 K for 90 min with glucose to catalyst weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 5, entry 1).
1 (Table 5, entry 1).
| Entry | Substrate | Solvent | Catalyst | Ta (K) | ta | Ca (%) | Ya (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
a T = temperature; t = time; C = conversion; Y = HMF yield.b The mole ratio of Ti/Zr was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.c Beta(OF)-Cal500 = organic structure-directing agent (OSDA)-free zeolite Beta calcined at 500 °C.d The pH value was 1 with HCl.e The mole ratio of Sn/glucose was 1 1.c Beta(OF)-Cal500 = organic structure-directing agent (OSDA)-free zeolite Beta calcined at 500 °C.d The pH value was 1 with HCl.e The mole ratio of Sn/glucose was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200.f Zr-MCM-550 was obtained from ZrO2/MCM-41 calcined at 550 °C.g The mole ratio of Si/Al was 10 or 5 in 10Al-MCM and 5Al-MCM, respectively.h FPIL 1a, FPIL 1b, FPIL 2a, and FPIL 2b were the functional polymeric ionic liquids (FPILs) by coupling of SO3H-FPILs with PW12O403− and Cl− counterpart anions without or with the treatment of CrCl3·6H2O, respectively.i PBnNH3Cl = an enzyme mimic ammonium polymer.j 80 μL concentrated HCl was added.k The structures were shown in Fig. 18, and the mole ratio of Cr/glucose was 3 200.f Zr-MCM-550 was obtained from ZrO2/MCM-41 calcined at 550 °C.g The mole ratio of Si/Al was 10 or 5 in 10Al-MCM and 5Al-MCM, respectively.h FPIL 1a, FPIL 1b, FPIL 2a, and FPIL 2b were the functional polymeric ionic liquids (FPILs) by coupling of SO3H-FPILs with PW12O403− and Cl− counterpart anions without or with the treatment of CrCl3·6H2O, respectively.i PBnNH3Cl = an enzyme mimic ammonium polymer.j 80 μL concentrated HCl was added.k The structures were shown in Fig. 18, and the mole ratio of Cr/glucose was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.l SBP = sec-butyl phenol.m The prepared methods for these catalysts were shown in ref. 113.n PCP = Cr-based porous coordination polymer, PTA = phosphotungstic acid. 10.l SBP = sec-butyl phenol.m The prepared methods for these catalysts were shown in ref. 113.n PCP = Cr-based porous coordination polymer, PTA = phosphotungstic acid. |
||||||||
| 1 | Glucose (0.15 g) | H2O (1.5 ml)–MIBK (3.5 ml) | Mesoporous tantalum oxide (50 mg) | 448 | 1.5 h | 69 | 23 | 96 |
| 2 | Glucose (2 g) | H2O (20 ml)–THF (80 ml)–NaCl (4 g) | TiO2–ZrO2 (0.8 g)b | 448 | 3 h | 97 | 71 | 97 |
| 3 | Glucose (2 g) | H2O (20 ml)–THF (80 ml)–NaCl (4 g) | TiO2–ZrO2 (0.4 g)b + Amberlyst 70 (0.4) | 448 | 3 h | 99.9 | 85.9 | 97 |
| 4 | Glucose (2 g) | H2O (20 ml)–THF (80 ml)–NaCl (4 g) | TiO2–ZrO2 (0.4 g)b + Nafion NR50 (0.4) | 448 | 3 h | 99.9 | 73.4 | 97 |
| 5 | Glucose (2 g) | H2O (20 ml)–THF (80 ml)–NaCl (4 g) | TiO2–ZrO2 (0.4 g)b + Cs2.5H0.5PW12O4 (0.4) | 448 | 3 h | 99.9 | 31.8 | 97 |
| 6 | Glucose (2 g) | H2O (20 ml)–THF (80 ml)–NaCl (4 g) | TiO2–ZrO2 (0.4 g)b + Cs3.5H0.5SiW12O4 (0.4) | 448 | 3 h | 99.9 | 35.2 | 97 |
| 7 | Cellulose (2 g) | H2O (20 ml)–THF (80 ml)–NaCl (4 g) | TiO2–ZrO2 (0.4 g)b + Amberlyst 70 (0.4) | 453 | 3 h | 42.1 | 25.5 | 97 |
| 8 | Glucose (0.67 mmol) | H2O (4.5 ml)–DMSO (0.5 ml)–THF (15 ml) | Beta(OF)-Cal500 (0.1 g)c | 453 | 3 h | 97 | 70 | 99 |
| 9 | Glucose (10 wt%)d | H2O (1 ml)–THF (3 ml)–NaCl (0.35 g) | Sn-Beta-Fe | 463 | 70 min | — | 53 | 100 |
| 10 | Glucose (0.15 g) | H2O (1.5 ml)–MIBK (3.5 ml) | Zr-MCM-550 (50 mg)f | 448 | 2.5 h | 82 | 23 | 101 |
| 11 | Glucose (0.15 g) | H2O (1.5 ml)–MIBK (3.5 ml)–NaCl (20 wt%) | 10Al-MCM (50 mg)g | 468 | 30 min | 98 | 63 | 103 |
| 12 | Glucose (0.15 g) | H2O (1.5 ml)–MIBK (3.5 ml) | 10Al-MCM (50 mg)g | 468 | 30 min | ∼87 | ∼36 | 103 |
| 13 | Glucose (0.15 g) | H2O (1.5 ml)–MIBK (3.5 ml) | 5Al-MCM (50 mg)g | 468 | 30 min | ∼71 | ∼21 | 103 |
| 14 | Glucose (0.1 g) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)/MIBK 4)/MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-BuOH (3 n-BuOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
FPIL 2a (30 mg)h | 423 | 2 h | — | 48.7 | 104 |
| 15 | Glucose (0.1 g) | DMSO (1 ml) | FPIL 2a (30 mg)h | 423 | 2 h | — | ∼34.5 | 104 |
| 16 | Glucose (0.1 g) | DMSO (1 ml) | FPIL 2b (30 mg)h | 423 | 2 h | — | ∼39.5 | 104 |
| 17 | Glucose (0.1 g) | DMSO (1 ml) | FPIL 1a (30 mg)h | 423 | 2 h | — | ∼18.5 | 104 |
| 18 | Glucose (0.1 g) | DMSO (1 ml) | FPIL 1b (30 mg)h | 423 | 2 h | — | ∼14.5 | 104 |
| 19 | Glucose (180 mg) | NaCl–saturated H2O (0.6 ml)–DMSO (1.4 ml)–MIBK (8 ml) | PBnNH3Cl (140 mg)i | 413 | 10 | ∼80 | 53 | 105 |
| 20 | Cellulose (162 mg) | NaCl–saturated H2O (0.6 ml)–DMSO (1.4 ml)–MIBK (8 ml) | PBnNH3Cl (140 mg)j | 433 | 2 | 100 | 40 | 105 |
| 21 | Glucose (5 wt%) | H2O (1.5 ml)–DMSO (0.5 ml)/MIBK (9.8 ml)–BuOH (4.2 ml) | Cr(III)-PDVB-0.3-SSFBIk | 413 | 7 | 94 | 60 | 106 |
| 22 | Glucose (5 wt%) | H2O (1.5 ml)–DMSO (0.5 ml)/MIBK (9.8 ml)–BuOH (4.2 ml) | Cr(III)-NKC-9k | 413 | 4 | 100 | ∼67 | 106 |
| 23 | Glucose (5 wt%) | H2O (1.5 ml)–DMSO (0.5 ml)/MIBK (9.8 ml)–BuOH (4.2 ml) | Cr(III)-PSFSI-MSMA15/SiO2k | 413 | 4 | 100 | ∼67 | 106 |
| 24 | Glucose (0.15 g) | H2O (1.5 ml)–MIBK (3.5 ml) | Mesoporous tantalum phosphate (50 mg) | 433 | 1 | 56.3 | 32.8 | 109 |
| 25 | Glucose (1 g) | THF (30 ml)–H2O (10 ml)–NaCl (3.5 g) | FePO4 (0.5 g) | 413 | 15 min | 97.8 | 23.1 | 110 |
| 26 | Cellulose (1 g) | THF (30 ml)–H2O (10 ml)–NaCl (3.5 g) | FePO4 (0.5 g) | 433 | 1 h | 87.4 | 48 | 110 |
| 27 | Glucose (1 g) | THF (30 ml)–H2O (10 ml)–NaCl (3.5 g) | CrPO4 (0.125 g) | 413 | 30 min | 99 | 63 | 111 |
| 28 | Glucose (2.4 g) | H2O (8 ml)–MIBK (18 ml) | Ag3PW12O40 (0.025 mmol) | 403 | 4 h | ∼87.5 | 76.3 | 112 |
| 29 | Glucose (5 wt%) | SBP/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) saturated with NaCll 1) saturated with NaCll |
Nb/CB-2-DP (0.1 g)m | 443 | 2 h | 78 | 20 | 113 |
| 30 | Glucose (5 wt%) | SBP/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) saturated with NaCl 1) saturated with NaCl |
Nb/CB-1-DP (0.1 g)m | 443 | 2 h | 34 | 18 | 113 |
| 31 | Glucose (5 wt%) | SBP/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) saturated with NaCl 1) saturated with NaCl |
Nb/CS-HT (0.1 g)m | 443 | 2 h | 33 | 11 | 113 |
| 32 | Glucose (75 mg) | THF–water (volume ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5 ml) 1, 1.5 ml) |
Na2ZrSi4O11 (200 mg) + Amberlyst-15 (50 mg) | 453 | 1.5 h | 87 | 39 | 114 |
| 33 | Glucose (0.1 g) | H2O (10 ml)–THF (20 ml)–NaCl (3.5 g) | PCP(Cr)-SO3H·Cr(III) (70 mg)n | 453 | 4 h | 100 | 80.7 | 115 |
| 34 | Glucose (0.1 g) | H2O (10 ml)–THF (20 ml)–NaCl (3.5 g) | PTA-PCP(Cr)-SO3H·Cr(III) (70 mg)n | 453 | 4 h | 100 | 45.3 | 115 |
Furthermore, the activity of TiO2–ZrO2 binary oxides for conversion of glucose to HMF was examined in the presence of some solid acids as the co-catalyst in water–THF biphasic reaction system.97 TiO2–ZrO2 with a Ti/Zr mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed the best performance due to the moderate acid–base concentration suitable for the glucose-to-HMF reaction. This cooperative interaction between the acid–base sites on TiO2–ZrO2 had also found in other reports, wherein glucose was isomerized to fructose on the basic sites of ZrO2 and subsequent dehydration of fructose to HMF on the acid sites of TiO2.51 Furthermore, the authors pointed out that glucose conversion and yield of HMF were not significantly enhanced in water–THF (a volume ratio of 1
1 showed the best performance due to the moderate acid–base concentration suitable for the glucose-to-HMF reaction. This cooperative interaction between the acid–base sites on TiO2–ZrO2 had also found in other reports, wherein glucose was isomerized to fructose on the basic sites of ZrO2 and subsequent dehydration of fructose to HMF on the acid sites of TiO2.51 Furthermore, the authors pointed out that glucose conversion and yield of HMF were not significantly enhanced in water–THF (a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). However, the addition of NaCl (20 wt% of the aqueous phase) into the THF/water solvent mixture could remarkably increase the HMF yield because NaCl promoted solvent partitioning into two phases: a reactive aqueous phase and an extractive organic phase, which allowed in situ extraction of HMF from the aqueous phase into the organic phase, thereby preventing undesired side reactions. In addition, the co-catalyst affected the activity significantly as followed: Amberlyst 70 > Nafion NR50 > Cs3.5SiW > Cs2.5PW, which was consistent with their acid strength. Because catalysts with stronger Brønsted acidity could improve HMF to huminic compounds, Amberlyst 70 with the lowest Brønsted acidity showed the best performance (Table 5, entries 2–6). The highest HMF yield of 85.9% could be obtained at 448 K for 3 h in THF–water (4/1 volume ratio) in the presence of NaCl catalyzed by TiO2–ZrO2 and Amberlyst (1/1 weight ratio). Meanwhile, this catalytic system could also be applied in conversion of cellulose to HMF at 453 K, and the HMF yield could reach 25.5% with a cellulose conversion of 42.1% (Table 5, entry 7). Further study found that a much higher HMF yield (86%) was achieved when the organic phase of the biphasic system was replaced with dioxane (Fig. 16), which may be ascribed to the role of dioxane as an aqueous phase modifier that stabilized HMF in the reactive phase as well as promoted partitioning of HMF into the extractive layer.
4). However, the addition of NaCl (20 wt% of the aqueous phase) into the THF/water solvent mixture could remarkably increase the HMF yield because NaCl promoted solvent partitioning into two phases: a reactive aqueous phase and an extractive organic phase, which allowed in situ extraction of HMF from the aqueous phase into the organic phase, thereby preventing undesired side reactions. In addition, the co-catalyst affected the activity significantly as followed: Amberlyst 70 > Nafion NR50 > Cs3.5SiW > Cs2.5PW, which was consistent with their acid strength. Because catalysts with stronger Brønsted acidity could improve HMF to huminic compounds, Amberlyst 70 with the lowest Brønsted acidity showed the best performance (Table 5, entries 2–6). The highest HMF yield of 85.9% could be obtained at 448 K for 3 h in THF–water (4/1 volume ratio) in the presence of NaCl catalyzed by TiO2–ZrO2 and Amberlyst (1/1 weight ratio). Meanwhile, this catalytic system could also be applied in conversion of cellulose to HMF at 453 K, and the HMF yield could reach 25.5% with a cellulose conversion of 42.1% (Table 5, entry 7). Further study found that a much higher HMF yield (86%) was achieved when the organic phase of the biphasic system was replaced with dioxane (Fig. 16), which may be ascribed to the role of dioxane as an aqueous phase modifier that stabilized HMF in the reactive phase as well as promoted partitioning of HMF into the extractive layer.
 | ||
Fig. 16 Influence of organic solvent in the biphase system on selective HMF yield with TiO2–ZrO2 (1/1) and Amberlyst 70 catalysts. Reaction conditions: 5 g glucose, 2 g catalyst weight (TiO2–ZrO2/Amberlyst = 1/1 w/w), 100 ml solvent (THF/water = 4/1 v/v), 4 g NaCl, 3 h reaction time, 448 K reaction temperature. ( ) Glucose conversion, ( ) Glucose conversion, ( ) HMForg and ( ) HMForg and ( ) HMFaq. Reproduced from ref. 97 with permission from the Royal Society of Chemistry. ) HMFaq. Reproduced from ref. 97 with permission from the Royal Society of Chemistry. | ||
6.2. Zeolites for dehydration of glucose and cellulose in biphasic solvent systems
Otomo and co-workers found that calcination and steam treatment of Beta zeolite could cleave a part of Si–O–Al bonds in the framework to form Al species out of the *BEA framework,98 which could increase the Lewis acid sites at the expense of Brønsted acid sites. Thus treated Beta zeolites were found to be efficient bifunctional catalysts for the synthesis of HMF from glucose in a biphasic system of water–THF. For example, Beta zeolite calcined at 1023 K showed 55% selectivity to HMF at 78% conversion of glucose. The authors clarified that the Lewis acid sites was beneficial for the isomerization of glucose to fructose, and the Brønsted acid sites promoted the dehydration of the generated fructose to HMF. In addition, the Beta zeolites could be recovered by filtration and regenerated by simple calcination, and the HMF yield remained fairly constant during five consecutive runs. Following this work, they recently found that organic structure-directing agent (OSDA)-free zeolite Beta with high Al content exhibited remarkably high catalytic performance in the conversion of glucose to HMF.99 Beta(OF) zeolite calcined at 773 K provided the best performance due to the existence of a sufficient number of Lewis acid sites and a large number of Brønsted acid sites for isomerization of glucose to fructose and subsequent dehydration of fructose to HMF.27 Al MAS NMR and IR spectroscopies indicated that Al species bearing OH groups were the active Lewis acid sites for the isomerization of glucose. Additionally, the solvent system was a key factor for the high yield of HMF with DMSO and THF because they could suppress the subsequent reactions of HMF into levulinic acid and humins. Finally, in water (4.5 ml)–DMSO (0.5 ml)–THF (15 ml) solvent system, a high HMF yield of about 70% could be produce over Beta(OF)-Cal500 at 453 K for 3 h (Table 5, entry 8).Sn-Beta zeolite was prepared with NH4F as the mineralizing agent (Sn-Beta-F) for conversion of glucose to HMF in water–THF solvent system.100 The obtained Sn-Beta-F needed to be combined with a Brønsted acid (HCl) to provide good activity for dehydration of glucose, because Sn-Beta-F was essential for isomerization of glucose to fructose while HCl was required for dehydration of fructose to HMF. In addition, the addition of NaCl could improve the selectivity of HMF due to the similar effect discussed above.89,97 A HMF yield of 53.0% could be obtained at 463 K with a reaction time of 70 min in water–THF system (volume ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) with 0.35 g NaCl added (Table 5, entry 9). In addition, cellulose could provide a HMF yield of 32.2% under the same conditions with glucose.
3) with 0.35 g NaCl added (Table 5, entry 9). In addition, cellulose could provide a HMF yield of 32.2% under the same conditions with glucose.
6.3. MCM-41-based solid catalysts for dehydration of glucose in biphasic solvent systems
In this content, Jiménez-Morales et al. conducted two works for dehydration of glucose to HMF in water–MIBK solvent system. Firstly, they prepared several mesoporous MCM-41 catalysts containing ZrO2 by three routes:101 (a) MCM-41 silica doped with Zr in the synthesis step and activated at 823 and 1023 K, (b) this solid impregnated with sulphuric acid and activated at 1023 K, and (c) MCM-41 impregnated with zirconium sulphate and calcined at 1023 K. The most active catalyst was the one obtained from incorporation of zirconium into a MCM-41 silica in the synthesis gel calcined at 823 K (Zr-MCM-550) due to the presence of both suitable Lewis and Brønsted acid sites for isomerization of glucose to fructose and subsequent dehydration of fructose to HMF.102 A HMF yield of 23 wt% could be achieved with a glucose of 82% at 448 K for 2.5 h in water (1.5 ml)–MIBK (3.5 ml) solvent system (Table 5, entry 10).In a following work, they prepared mesoporous aluminium doped MCM-41 silica catalysts by a sol–gel method.103 Two solids with different Si/Al molar ratio (5 and 10) were obtained, denoted as 5Al-MCM and 10Al-MCM. For dehydration of glucose, 10Al-MCM showed better performance than 5Al-MCM (Table 5, entries 12 and 13) because 10Al-MCM had a higher amount of strong acid sites (both Brønsted and Lewis types) while the presence of amorphous alumina in 5Al-MCM could block the pores where the most acidic sites were located, thus leading to a reduced catalytic activity. Meanwhile, the addition of 20 wt% NaCl could improve the HMF yield due to that salting-out effect of NaCl favored HMF in the extracting phase, which could decrease the decomposition of HMF. Over 10Al-MCM, a HMF yield of 63% was achieved with a glucose conversion of 98% at 468 K in 30 min in water (1.5 ml)–MIBK (3.5 ml) solvent system with the presence of 20 wt% NaCl (Table 5, entry 11).
6.4. Functional polymers for dehydration of glucose and cellulose in biphasic solvent systems
A series of functional polymeric ionic liquids (FPILs) were prepared by coupling of SO3H-FPILs with PW12O403− and Cl− counterpart anions without or with the treatment of CrCl3·6H2O, denoted as FPIL 1a, FPIL 1b, FPIL 2a, and FPIL 2b, respectively.104 FPIL 2a and FPIL 2b showed better performance than FPIL 1a and FPIL 1b for dehydration of glucose because of the lack of active sites (Cr3+) in FPIL 1a and FPIL 1b for the isomerization of glucose to fructose (Table 5, entries 15–18), indicating the synergistic effect of Cr3+ and SO3H group. In H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1
DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)/MIBK
4)/MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-BuOH (3
n-BuOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) solvent system, a HMF yield of 48.7% was obtained at 423 K for 2 h catalyzed by FPIL 2a (Table 5, entry 14). In addition, FPIL 2a could be easily recycled for at least five times without significant loss of activity.
7) solvent system, a HMF yield of 48.7% was obtained at 423 K for 2 h catalyzed by FPIL 2a (Table 5, entry 14). In addition, FPIL 2a could be easily recycled for at least five times without significant loss of activity.
An enzyme mimic ammonium polymer (PBnNH3Cl) was synthesized by Cao et al. and used as heterogeneous catalyst for dehydration of glucose in a biphasic reaction system.105 A maximum HMF yield of 53% could be obtained at 413 K for 10 h in NaCl–saturated water (0.6 ml)–DMSO (1.4 ml)–MIBK (8 ml) solvent system, while the yield of HMF was about 39% in pure DMSO at 393 K for 10 h (Table 5, entry 19). In the catalytic cycle (Fig. 17), PBnNH3Cl worked as a dual functional organocatalyst for glucose isomerization and subsequent dehydration. Furthermore, this catalyst could catalyze dehydration of cellulose with a HMF yield of 40% produced in the same solvent system at 433 K for 2 h (Table 5, entry 20).
 | ||
| Fig. 17 Reaction pathways for glucose dehydration in the PBnNH3Cl/DMSO system. Reproduced from ref. 105 with permission from the Royal Society of Chemistry. | ||
Very recently, three bifunctional catalysts (Cr(III)-NKC-9, Cr(III)-PDVB-0.3-SSFBI and Cr(III)-PSFSI-MSMA15/SiO2) were synthesized by the ion exchange between CrCl3 and NKC-9 (a SO3H-style resin with polystyrene as support), H-PDVB-0.3-SSFBI (hydrophobic poly-divinylbenzene (PDVB) polymer with fluoroalkyl sulfonyl imide groups), and H-PSFSI-MSMA15/SiO2, respectively (Fig. 18).106 These catalysts could be applied in dehydration of glucose in water–MIBK solvent system. Cr(III)-PDVB-0.3-SSFBI showed a lower activity than the other two catalyst due to its lower promotion of glucose isomerization to fructose. However, Cr(III)-PDVB-0.3-SSFBI showed better recyclability due to its integral hydrophobicity with water-tolerance of SO2NHSO2C4F9 groups (Table 5, entries 21–23). In water (1.5 ml)–DMSO (0.5 ml)/MIBK (9.8 ml)–n-BuOH (4.2 ml), a HMF yield of about 60% could be achieved at 413 K for 7 h.
 | ||
| Fig. 18 Chemical structures of solid Brønsted acids. Reproduced from ref. 106 with permission from the Royal Society of Chemistry. | ||
6.5. Various metal phosphates for dehydration of glucose and cellulose in biphasic solvent systems
Metal phosphates are a kind of organic–inorganic hybrid material with multifunctionality, exhibiting potential applications in ion exchange, adsorption, photochemistry, and especially in catalysis. Due to the insolubility in most solvents, metal phosphates have been considered as an important class of heterogeneous catalysts. For dehydration of glucose and cellulose, several metal phosphates have been used as efficient catalysts in biphasic solvent systems.The activity of several metal phosphates, including aluminum (AlPO), titanium (TiPO), zirconium (ZrPO), and niobium phosphates (NbPO) was examined by Ordomsky et al. for dehydration of glucose in water–MIBK or water-2-methyltetrahydrofuran (MTHF) solvent system.107 The activity of these metal phosphates decreased in the order: NbPO > ZrPO > TiPO > AlPO, which was consistent with the amount of strong acid sites on these catalysts. Meanwhile, the ratio of Brønsted to Lewis acid sites affected the selectivity, and excess of Lewis acidity led to the formation of humins. Modification of the catalysts by a silylation procedure or by deeper treatment with phosphoric acid leads to drastic increase in the selectivity to HMF due to the deactivation of unselective Lewis acid sites, and the highest selectivity to HMF was found to be 55–60% over silylated phosphates. The authors thought that a synergism of a protonated phosphate group and a nearby metal Lewis acid site led to a highly selective glucose dehydration to HMF in the biphasic solvent systems. In another work, ZrPO was coated on aluminum foam to form a foam-structured catalyst for glucose dehydration in a biphasic system (water–MIBK) by the same authors.108 ZrPO foam-based catalyst showed lower activity than the bulk ZrPO due to the shorter contact time between the solid foam and the aqueous phase, in which the reaction happened. However, these catalysts demonstrated a higher isomerization activity of glucose to fructose. Furthermore, a silylation treatment could result in a higher HMF selectivity due to the deactivation of unselective Lewis acid sites (Fig. 19). In these two works, the authors found that a high Brønsted/Lewis acid molar ratio could promote the formation of HMF from glucose, while a low Brønsted/Lewis acid molar ratio (ca. 0.09–0.27) would inhibit the formation of humins or oligomers.107 However, an excess higher Brønsted/Lewis acid molar ratio (ca. 0.72–1.85) would decrease the selectivity to HMF.108 Therefore, suitable Brønsted/Lewis acid ratio played an important role to achieve higher yield and selectivity of HMF from glucose.
 | ||
| Fig. 19 Glucose dehydration over parent ZrPO (left) and that after silylation (right). Reproduced from ref. 108 with permission from John Wiley and Sons. | ||
Mesoporous tantalum phosphate from tantalum tartrate and ammonium phosphate monobasic was synthesized in the presence of an ionic surfactant, and subsequent calcined at 823 K.109 The obtained tantalum phosphate was used to catalyze the dehydration of glucose in water, water–MIBK, and water–methyl isobutylether systems. Among them, water–MIBK system showed the best performance due to the high value for the HMF partition coefficient in it. A HMF yield of 32.8% could be produced with a glucose conversion of 56.3% at 443 K for 1 h in water (1.5 g)–MIBK (3.5 ml) system (Table 5, entry 24). The authors thought that the high catalytic activity of this used solid catalyst could be associated to its high acidity and the presence of both Brønsted and Lewis acid sites, which were maintained in water.
Recently, commercial FePO4 was used as catalyst for transformation of glucose and cellulose to HMF in a THF–H2O biphasic system with the presence of NaCl in Xia's group.110 As shown in Fig. 20, FePO4 combined the characteristics of both homogeneous and heterogeneous catalysts, which could act as a dissolved homogenous acid at higher temperature and as an insoluble solid acid after cooling to room temperature. In the reaction cycle, Fe3+ acted as a Lewis acid catalyst for the isomerization of glucose into fructose, while H3PO4 generated from the hydrolysis of FePO4 catalyzed the subsequent dehydration of fructose into HMF. A HMF yield of 23.1% could be generated from glucose at 413 K for 15 min in THF (30 ml)–water (10 ml) system in the presence of NaCl (3.5 g) (Table 5, entry 25). Meanwhile, FePO4 was also effective for HMF production from cellulose with a HMF yield of 48% at 433 K for 60 min in the same solvent system with glucose (Table 5, entry 26). Subsequently, they also found CrPO4 was also effective for dehydration of cellulose and glucose to HMF in the above-mentioned solvent system.111 A maximum HMF yield of 63% could be obtained at 413 K for 30 min, and cellulose could produce 37% HMF at 413 K for 15 min (Table 5, entry 27). The authors proposed a possible reaction route for CrPO4-catalyzed conversion of cellulose involving the homogeneous acid-catalyzed depolymerization of cellulose to glucose, a Lewis acid site [Cr(H2O)5OH]2+ (generated from the hydrolysis of CrPO4)-catalyzed isomerization of glucose to fructose, and a homogeneous acid (H+ from the hydrolysis of CrPO4)-catalyzed dehydration of fructose to HMF (Fig. 21). However, it should be pointed out that this catalytic system may be a homogeneous one.
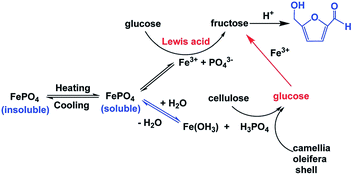 | ||
| Fig. 20 Proposed reaction pathways of FePO4 catalyzed dehydration of glucose to produce HMF. Reproduced from ref. 110 with permission from the Royal Society of Chemistry. | ||
 | ||
| Fig. 21 Proposed reaction pathways of CrPO4 catalyzed conversion of cellulose to HMF. Reproduced from ref. 111 with permission from the Royal Society of Chemistry. | ||
6.6. Other heterogeneous catalysts for dehydration of glucose and cellulose in biphasic solvent systems
Solid heteropolyacid salt Ag3PW12O40 was prepared by the ion exchange between H3PW12O40 and AgNO3, which could be used as heterogeneous catalyst for dehydration of glucose in water–MIBK solvent system.112 Ag3PW12O40 showed better performance than HCl, H3PW12O40, and AgNO3 due to the synergistic effect of Lewis acid sites and Brønsted acid sites and the accumulation of glucose on it. A HMF yield of 76.3% could be gained at 403 K for 4 h in water (8 ml)–MIBK (18 ml) system (Table 5, entry 28). In addition, this solid acidic catalyst was stable and could be reused at least six reaction cycles.Three niobia/carbon acid catalysts were designed, including Nb/CB-1-DP (carbon black was treated at 353 K and niobia was subsequently loaded by homogeneous deposition precipitation), Nb/CB-2-DP (carbon black was treated at 393 K and niobia was subsequently loaded by homogeneous deposition precipitation), and Nb/CS-HT (prepared by deposition precipitation-carbonization using glucose as the carbon source).113 Among them, the latter catalyst provided crystalline niobia particles, while the other catalysts yielded amorphous niobia. These catalysts could catalyze dehydration of glucose to HMF in water-sec-butyl phenol (SBP) solvent system. The three catalysts showed different activity because they were located in different phase (Nb/CB-1-DP in organic phase, Nb/CB-2-DP in aqueous phase, and Nb/CS-HT at the interface of the two phases) resulting from their different hydrophobicity/hydrophilicity, and Nb/CB-2-DP showed the best performance with a HMF yield of 20% at 443 K for 2 h in SBP–water (saturated with NaCl, SBP/water volume was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system (Table 5, entries 29–31).
1) system (Table 5, entries 29–31).
The combination of layered zirconosilicate Na2ZrSi4O11 and Amberlyst-15 could be used as heterogeneous catalysts for dehydration of glucose to HMF.114 The HMF yield could reach 39% with a glucose conversion of 87% at 453 K for 1.5 h in THF–water (volume ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system (Table 5, entry 32). Na2ZrSi4O11 acted as a basic catalyst for isomerization of glucose to fructose, and Amberlyst-15, a solid acid catalyst, catalyzed the subsequent dehydration of the generated fructose to HMF.
1) system (Table 5, entry 32). Na2ZrSi4O11 acted as a basic catalyst for isomerization of glucose to fructose, and Amberlyst-15, a solid acid catalyst, catalyzed the subsequent dehydration of the generated fructose to HMF.
Recently, Cr-based porous coordination polymers (PCPs) modified with sulfonic acid (PCP(Cr)-SO3H·Cr(III)) or phosphotungstic acid (PTA, PTA-PCP(Cr)-SO3H·Cr(III)) were prepared by hydrothermal method,115 and used as solid acidic catalysts for dehydration of glucose to HMF in water–THF solvent system. PCP(Cr)-SO3H·Cr(III) had better performance than PTA-PCP(Cr)-SO3H·Cr(III) (Table 5, entries 33–34), and a HMF yield of 80.7% was achieved at 453 K for 4 h in water (10 ml)–THF (20 ml) system with the presence of 3.5 g NaCl to increase immiscibility between water and THF phase, while the yield was only 45.3% over PTA-PCP(Cr)-SO3H·Cr(III) resulted from the blockage of pores by the encapsulated PTA. The catalytic cycle involved two steps: the first step was the isomerization of glucose to fructose on the Cr Lewis acid sites, and the second step was the dehydration of fructose to HMF mainly on the sulfonic group Brønsted acid sites (Fig. 22).
 | ||
| Fig. 22 Conversion of glucose to HMF catalyzed by PCP(Cr)-SO3H·Cr(III) in water–THF biphasic system. Reproduced from ref. 115 with permission from Elsevier. | ||
7. Conclusion and outlook
Efficient and selective conversion of glucose and cellulose to HMF over heterogeneous catalysts is an important topic in the field of biomass transformation. In recent years, significant advances have been made for conversion of glucose and cellulose to HMF, and many kinds of effective heterogeneous catalysts have been developed for this important conversion in various solvent systems, including water, organic solvents, ionic liquids, mixed solvents systems, and biphasic solvent systems. The activity and selectivity of the catalysts for heterogeneous dehydration of glucose and cellulose vary from the solvent systems. (1) Generally, lower yield of HMF was generated from heterogeneous dehydration of glucose and cellulose in water because of the side-reactions (e.g. hydrolysis, the formation of humin) of HMF in water with the existence of acidic catalysts and the low solubility of cellulose in water,51,52 except for some special catalysts.52,53 (2) Compared with the reactions in water, heterogeneous dehydration of glucose in organic solvents could generate higher yield of HMF due to the inhibiting effect of these organic solvents on the side-reaction to some extent.66,70,71 However, the examples for conversion of cellulose in organic solvents over heterogeneous catalysts was very little with a low yield of HMF resulting from the low solubility of cellulose in organic solvents.70 (3) As a novel green solvents, ionic liquids provide an effective class of solvents for dehydration of glucose77,78,80,81,86,88 and cellulose78,79,82–84,86 over various heterogeneous catalysts due to their good solubility of glucose and cellulose and their effect on stability of intermediates. (4) Some kinds of heterogeneous catalysts showed high activity and selectivity in monophasic mixed solvent systems because of the regulation of the solvent properties by mixing two or more solvents, and high yield of HMF could be obtained from glucose,89–94 even cellulose.89,91 (5) For heterogeneous dehydration of glucose and cellulose, biphasic solvents systems can be recognized as one of the most effective solvent systems, and high yield of HMF could be achieved because of the in situ separation of the generated HMF from the reaction phase (Table 5),96,97,100,101,103–106,109–115 which can decrease the side-reactions and the decomposition of HMF, and thus increasing the selectivity of HMF ultimately.Furthermore, it should be pointed out that the Lewis acidic sites and the Brønsted acidic sites play different role for conversion of cellulose and glucose to HMF. Generally, Brønsted acidic sites can promote the hydrolysis of cellulose and dehydration of fructose, which are the first and third step of conversion of cellulose as shown in Fig. 2. In contrast, the Lewis acidic sites can play important role in the three steps of cellulose conversion, especially for the isomerization of glucose to fructose, which is the most important step in the transformation of cellulose and glucose to HMF. More importantly, it has been found that suitable Brønsted/Lewis acid ratio is important to achieve higher yield and selectivity of HMF from cellulose and glucose.107,108,116 Therefore, efforts should be devoted to determining the detail role of Brønsted and Lewis acid sites in the conversion of glucose and cellulose to HMF.
Overall, this review provides a holistic overview of the developed heterogeneous catalysts for HMF production from dehydration of glucose and cellulose. Based on the reported works for this important conversion, it is no doubt that development of heterogeneous catalysts for conversion of glucose and cellulose to HMF is very promising and encouraging. However, there are many challenges need to be addressed, and we would like to discuss some of them.
(1) Some of the used heterogeneous catalysts were not stable, especially under high reaction temperatures. Design of novel stable heterogeneous catalysts with high catalytic activity for dehydration of glucose and cellulose is very crucial.
(2) Multi-functional heterogeneous catalysts should be further developed for the tandem conversion of glucose and cellulose to HMF. For example, HMF production involves hydrolysis, isomerization, and dehydration from cellulose or isomerization and dehydration from glucose. Therefore, catalysts having multi-catalytic sites simultaneously might show better activity and selectivity. Much attention should be paid on this aspect. Additionally, Brønsted acid and Lewis acid of the heterogeneous catalysts should be examined (by infrared spectra of pyridine adsorption) and controlled in suitable amount and strength because the conversion of cellulose and glucose and the selectivity of HMF are affected significantly by their amount, strength and ratio.
(3) In some extent, solvents play the key role on the activity and selectivity. New functional solvent systems should be developed for the conversion of glucose and cellulose to HMF.
(4) Generally, the performance of the catalysts is affected by the solvents significantly. Hence, in order to achieve high yield of HMF, the coupling design of catalysts and solvent systems is another key issue to be taken into account.
(5) To understand the mechanisms for conversion of glucose and cellulose to HMF under various catalysts and solvents deeply is also a crucial and necessary aspect. Efforts should be paid to understand the molecular interactions between the feedstock, catalyst and solvents, and finally make clear their specific function for dehydration of cellulose and glucose to HMF with high activity and selectivity, which is helpful for the design of novel heterogeneous catalysts and solvent systems.
(6) How to suppress the side reactions during the conversion of glucose and cellulose to HMF is another important issue. Two aspects may be considered for this problem. Firstly, design novel solvent systems, e.g., biphasic solvent systems and mixed solvent systems, to change the solvent nature (viscosity, polarity), which can be helpful for the mass transfer of HMF from the catalyst surface to solvents. This effect can decrease the contact time between HMF and the catalytic active sites, and thus suppress the side reactions. Secondly, modifying the surface (hydrophilicity/hydrophobicity) of the catalyst can tune the interaction between HMF and the catalyst. Proper surface nature could decrease the above-mentioned interaction, and thus decrease the occurrence of the side reactions.
(7) Isolation of HMF from the reaction system is also a very important problem in industry. However, this aspect has not attracted much attention in current research works. More efforts should be devoted to design and establish efficient methods for HMF isolation targeted for different reaction systems, which will be beneficial for the commercial production and further utilization of HMF from glucose and cellulose.
Acknowledgements
The authors thank the National Natural Science Foundation of China (21503016, 21473252) for financial support.Notes and references
- National Twelfth Five-Year Plan on biomass energy, Chinese National Energy Administration, Beijing, 2012 Search PubMed.
- IRENA, REmap 2030: A Renewable Energy Roadmap, June 2014, IRENA, Abu Dhabi, 2014, http://irena.org/remap/ Search PubMed.
- USDA Announces Investments in Bioenergy Research and Development to Spur New Markets, Innovation, and Unlimited Opportunity in Rural America, United States Department of Agriculture, 2013 Search PubMed.
- C. Chatterjee, F. Pong and A. Sen, Green Chem., 2015, 17, 40–71 RSC.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Chem. Soc. Rev., 2012, 41, 8075–8098 RSC.
- J. S. Luterbacher, D. M. Alonso and J. A. Dumesic, Green Chem., 2014, 16, 4816–4838 RSC.
- J. L. Sawin and F. Sverrisson, Renewables 2014: Global status report, REN21 secretariat, Paris, 2014 Search PubMed.
- Z. H. Yuan, W. Luo, P. W. Lv, Z. M. Wang and H. W. Li, Chem. Ind. Eng. Prog., 2009, 28, 1687–1692 Search PubMed.
- T. A. Werpy and G. Petersen, Top value added chemicals from biomass, U.S. Department of Energy (DOE), DOE/GO-102004–1992, Golden, CO, 2004 Search PubMed.
- R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- X. Han, L. Geng, Y. Guo, R. Jia, X. Liu, Y. Zhang and Y. Wang, Green Chem., 2016, 18, 1597–1604 RSC.
- R. Fang, R. Luque and Y. Li, Green Chem., 2016, 18, 3152–3157 RSC.
- J. Chen, R. Liu, Y. Guo, L. Chen and H. Gao, ACS Catal., 2015, 5, 722–733 CrossRef CAS.
- G.-H. Wang, J. Hilgert, F. H. Richter, F. Wang, H.-J. Bongard, B. Splietho, C. Weidenthaler and F. Schüth, Nat. Mater., 2014, 13, 294–301 Search PubMed.
- Q.-N. Xia, Q. Cuan, X.-H. Liu, X.-Q. Gong, G.-Z. Lu and Y.-Q. Wang, Angew. Chem., Int. Ed., 2014, 53, 9755–9760 CrossRef CAS PubMed.
- Y. Shen, J.-K. Sun, Y.-X. Yi, B. Wang, F. Xu and R.-C. Sun, Bioresour. Technol., 2015, 192, 812–816 CrossRef CAS PubMed.
- B. Kim, J. Jeong, D. Lee, S. Kim, H.-J. Yoon, Y.-S. Lee and J. K. Cho, Green Chem., 2011, 13, 1503–1506 RSC.
- L. Hu, L. Lin, Z. Wu, S. Zhou and S. Liu, Appl. Catal., B, 2015, 174–175, 225–243 CrossRef CAS.
- J. Wang, J. Xi and Y. Wang, Green Chem., 2015, 17, 737–751 RSC.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- G. Yong, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS PubMed.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- T. Ståhlberg, M. G. Sørensen and A. Riisager, Green Chem., 2010, 12, 321–325 RSC.
- T. Deng, X. Cui, Y. Qi, Y. Wang, X. Hou and Y. Zhu, Chem. Commun., 2012, 48, 5494–5496 RSC.
- Y. Su, H. M. Brown, X. Huang, X. Zhou, J. E. Amonette and Z. C. Zhang, Appl. Catal., A, 2009, 361, 117–122 CrossRef CAS.
- J. Shi, H. Gao, Y. Xia, W. Li, H. Wang and C. Zheng, RSC Adv., 2013, 3, 7782–7790 RSC.
- B. R. Caes, M. J. Palte and R. T. Raines, Chem. Sci., 2013, 4, 196–199 RSC.
- Y. Yang, C.-W. Hu and M. M. Abu-Omar, Green Chem., 2012, 14, 509–513 RSC.
- Z.-D. Ding, W.-X. Gu, J.-J. Xiao and H.-J. Wang, Carbohydr. Polym., 2012, 90, 792–798 CrossRef CAS PubMed.
- C. Li, Z. Zhang and Z. K. Zhao, Tetrahedron Lett., 2009, 50, 5403–5405 CrossRef CAS.
- Y. Qu, C. Huang, Y. Song, J. Zhang and B. Chen, Bioresour. Technol., 2012, 121, 462–466 CrossRef CAS PubMed.
- H. Matsumiya and T. Hara, Biomass Bioenergy, 2015, 72, 227–232 CrossRef CAS.
- A. Chinnappan, A. H. Jadhav, W.-J. Chung and H. Kim, Ind. Crops Prod., 2015, 76, 12–17 CrossRef CAS.
- L. Wu, J. Song, B. Zhang, B. Zhou, H. Zhou, H. Fan, Y. Yang and B. Han, Green Chem., 2014, 16, 3935–3941 RSC.
- L. Hu, Y. Sun and L. Lin, Ind. Eng. Chem. Res., 2012, 51, 1099–1104 CrossRef CAS.
- Y.-M. Lu, H. Li, J. He, Y.-X. Liu, Z.-B. Wu, D.-Y. Hu and S. Yang, RSC Adv., 2016, 6, 12782–12787 RSC.
- J. Li, Y. Ma, L. Wang, Z. Song, H. Li, T. Wang, H. Li and W. Eli, Catalysts, 2016, 6, 1 CrossRef CAS.
- (a) S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745–4755 CrossRef CAS; (b) I. van Zandvoort, Y. Wang, C. B. Rasrendra, E. R. H. van Eck, P. C. A. Bruijnincx, H. J. Heeres and B. M. Weckhuysen, ChemSusChem, 2013, 6, 1745–1758 CrossRef CAS PubMed; (c) Z. Zhang, Q. Wang, H. Xie, W. Lu and Z. K. Zhao, ChemSusChem, 2011, 4, 131–138 CrossRef CAS PubMed.
- (a) M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS PubMed; (b) P. Zhou and Z. Zhang, Catal. Sci. Technol., 2016, 6, 3694–3712 RSC.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- B. Saha and M. M. Abu-Omar, Green Chem., 2014, 16, 24–38 RSC.
- S. Dutta, S. De and B. Saha, Biomass Bioenergy, 2013, 55, 355–369 CrossRef CAS.
- C. I. Herrerías, X. Yao, Z. Li and C.-J. Li, Chem. Rev., 2007, 107, 2546–2562 CrossRef PubMed.
- R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed.
- U. M. Lindström, Chem. Rev., 2002, 102, 2751–2772 CrossRef.
- M. Watanabe, Y. Aizawa, T. Iida, T. M. Aida, C. Levy, K. Sue and H. Inomata, Carbohydr. Res., 2005, 340, 1925–1930 CrossRef CAS PubMed.
- M. Watanabe, Y. Aizawa, T. Iida, R. Nishimura and H. Inomata, Appl. Catal., A, 2005, 295, 150–156 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Catal. Commun., 2008, 9, 2244–2249 CrossRef CAS.
- A. Chareonlimkun, V. Champreda, A. Shotipruk and N. Laosiripojana, Fuel, 2010, 89, 2873–2880 CrossRef CAS.
- Y. Li and J. Yu, Chem. Rev., 2014, 114, 7268–7316 CrossRef CAS PubMed.
- A. Primo and H. Garcia, Chem. Soc. Rev., 2014, 43, 7548–7561 RSC.
- K. Y. Nandiwale, N. D. Galande, P. Thakur, S. D. Sawant, V. P. Zambre and V. V. Bokade, ACS Sustainable Chem. Eng., 2014, 2, 1928–1932 CrossRef CAS.
- X. S. Zhao, G. Q. Lu and G. J. Millar, Ind. Eng. Chem. Res., 1996, 35, 2075–2090 CrossRef CAS.
- C.-W. Jiang, X. Zhong and Z.-H. Luo, RSC Adv., 2014, 4, 15216–15224 RSC.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171–198 CrossRef CAS PubMed.
- Y.-F. Song and R. Tsunashima, Chem. Soc. Rev., 2012, 41, 7384–7402 RSC.
- S. S. Wang and G.-Y. Yang, Chem. Rev., 2015, 115, 4893–4962 CrossRef CAS PubMed.
- S. Zhao, M. Cheng, J. Li, J. Tian and X. Wang, Chem. Commun., 2011, 47, 2176–2178 RSC.
- H. Pan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef.
- J. Liu, Y.-C. Liu, Y.-M. Lu, J. He, X.-F. Liu, Z.-B. Wu and S. Yang, Catal. Commun., 2015, 62, 19–23 CrossRef CAS.
- M. Hara, T. Yoshida, A. Takagaki, T. Takata, J. N. Kondo, S. Hayashi and K. Domen, Angew. Chem., Int. Ed., 2004, 43, 2955–2958 CrossRef CAS PubMed.
- A. Stein, Z. Wang and M. A. Fierke, Adv. Mater., 2009, 21, 265–293 CrossRef CAS.
- R. S. Thombal and V. H. Jadhav, Appl. Catal., A, 2015, 499, 213–216 CrossRef CAS.
- L. Vilcocq, P. C. Castilho, F. Carvalheiro and L. C. Duarte, ChemSusChem, 2014, 7, 1010–1019 CrossRef CAS PubMed.
- R. Sahu and P. L. Dhepe, ChemSusChem, 2012, 5, 751–761 CrossRef CAS PubMed.
- J. A. Bootsma and B. H. Shanks, Appl. Catal., A, 2007, 327, 44–51 CrossRef CAS.
- Y. Wang, Z. Gu, W. Liu, Y. Yao, H. Wang, X.-F. Xia and W. Li, RSC Adv., 2015, 5, 60736–60744 RSC.
- M. Ohara, A. Takagaki, S. Nishimura and K. Ebitani, Appl. Catal., A, 2010, 383, 149–155 CrossRef CAS.
- H. Li, X. He, Q. Zhang, F. Chang, W. Xue, Y. Zhang and S. Yang, Energy Technol., 2013, 1, 151–156 CrossRef CAS.
- V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615–2665 CrossRef PubMed.
- A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed.
- S. Zhang, J. Sun, X. Zhang, J. Xin, Q. Miao and J. Wang, Chem. Soc. Rev., 2014, 43, 7838–7869 RSC.
- C. Maton, N. De Vos and C. V. Stevens, Chem. Soc. Rev., 2013, 42, 5963–5977 RSC.
- Z. Zhang and Z. K. Zhao, Bioresour. Technol., 2011, 102, 3970–3972 CrossRef CAS PubMed.
- Z. Fang, B. Liu, J. Luo, Y. Ren and Z. Zhang, Biomass Bioenergy, 2014, 60, 171–177 CrossRef CAS.
- Y. Zhang, J. Pan, M. Gan, H. Qu, Y. Yan, W. Shi and L. Yu, RSC Adv., 2014, 4, 11664–11672 RSC.
- B. Liu, C. Ba, M. Jin and Z. Zhang, Ind. Crops Prod., 2015, 76, 781–786 CrossRef CAS.
- H. Liu, H. Wang, Y. Li, W. Yang, C. Song, H. Li, W. Zhu and W. Jiang, RSC Adv., 2015, 5, 9290–9297 RSC.
- Y. Zhang, J. Pan, Y. Yan, W. Shi and L. Yu, RSC Adv., 2014, 4, 23797–23806 RSC.
- Y. Zhang, J. Pan, Y. Shen, W. Shi, C. Liu and L. Yu, ACS Sustainable Chem. Eng., 2015, 3, 871–879 CrossRef CAS.
- Y. Zhang, Y. Chen, Y. Shen, Y. Yan, J. Pan, W. Shi and L. Yu, ChemPlusChem, 2016, 81, 108–118 CrossRef CAS.
- H. Gao, Y. Peng, J. Pan, J. Zeng, C. Song, Y. Zhang, Y. Yan and W. Shi, RSC Adv., 2014, 4, 43029–43038 RSC.
- L. Hu, Z. Wu, J. Xu, Y. Sun, L. Lin and S. Liu, Chem. Eng. J., 2014, 244, 137–144 CrossRef CAS.
- Q. Xu, Z. Zhu, Y. Tian, J. Deng, J. Shi and Y. Fu, BioResources, 2014, 9, 303–315 CAS.
- L. Hu, G. Zhao, X. Tang, Z. Wu, J. Xu, L. Lin and S. Liu, Bioresour. Technol., 2013, 148, 501–507 CrossRef CAS PubMed.
- J. Wang, J. Ren, X. Liu, J. Xi, Q. Xia, Y. Zu, G. Lu and Y. Wang, Green Chem., 2012, 14, 2506–2512 RSC.
- F. Guo, Z. Fang and T.-J. Zhou, Bioresour. Technol., 2012, 112, 313–318 CrossRef CAS PubMed.
- Z. Zhang, B. Du, L.-J. Zhang, Y.-X. Da, Z.-J. Quan, L.-J. Yang and X.-C. Wang, RSC Adv., 2013, 3, 9201–9205 RSC.
- F. D'Anna, S. Marullo, P. Vitale, C. Rizzo, P. L. Meo and R. Noto, Appl. Catal., A, 2014, 482, 287–293 CrossRef.
- L. Li, J. Ding, J.-G. Jiang, Z. Zhu and P. Wu, Chin. J. Catal., 2015, 36, 820–828 CrossRef CAS.
- Y. Wang, Y. Liu, L. Yang, R. Ruan, P. Wen and Y. Wan, Synth. React. Inorg., Met.–Org., Nano-Met. Chem., 2016, 46, 177–184 CrossRef CAS.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef PubMed.
- I. Jiménez-Morales, M. Moreno-Recio, J. Santamaría-González, P. Maireles-Torres and A. Jiménez-López, Appl. Catal., B, 2014, 154–155, 190–196 CrossRef.
- L. Atanda, A. Silahua, S. Mukundan, A. Shrotri, G. Torres-Torres and J. Beltramini, RSC Adv., 2015, 5, 80346–80352 RSC.
- R. Otomo, T. Yokoi, J. N. Kondo and T. Tatsumi, Appl. Catal., A, 2014, 470, 318–326 CrossRef CAS.
- R. Otomo, T. Yokoi and T. Tatsumi, ChemCatChem, 2015, 7, 4180–4187 CrossRef CAS.
- G. Yang, C. Wang, G. Lyu, L. A. Lucia and J. Chen, BioResources, 2015, 10, 5863–5875 CAS.
- I. Jiménez-Morales, J. Santamaría-González, A. Jiménez-López and P. Maireles-Torres, Fuel, 2014, 118, 265–271 CrossRef.
- E. Rodríguez-Castellón, A. Jiménez-López, P. Maireles-Torres, D. J. Jones, J. Rozière, M. Trombetta, G. Busca, M. Lenarda and L. Storaro, J. Solid State Chem., 2003, 175, 159–169 CrossRef.
- I. Jiménez-Morales, M. Moreno-Recio, J. Santamaría-González, P. Maireles-Torres and A. Jiménez-López, Appl. Catal., B, 2015, 164, 70–76 CrossRef.
- H. Li, Q. Zhang, X. Liu, F. Chang, Y. Zhang, W. Xue and S. Yang, Bioresour. Technol., 2013, 144, 21–27 CrossRef CAS PubMed.
- X. Cao, S. P. Teong, D. Wu, G. Yi, H. Su and Y. Zhang, Green Chem., 2015, 17, 2348–2352 RSC.
- X. Wang, H. Zhang, J. Ma and Z.-H. Ma, RSC Adv., 2016, 6, 43152–43158 RSC.
- V. V. Ordomsky, V. L. Sushkevich, J. C. Schouten, J. van der Schaaf and T. A. Nijhuis, J. Catal., 2013, 300, 37–46 CrossRef CAS.
- V. V. Ordomsky, J. van der Schaaf, J. C. Schouten and T. A. Nijhuis, ChemSusChem, 2013, 6, 1697–1707 CrossRef CAS PubMed.
- I. Jiménez-Morales, A. Teckchandani-Ortiz, J. Santamaría-González, P. Maireles-Torres and A. Jiménez-López, Appl. Catal., B, 2014, 144, 22–28 CrossRef.
- L. Yang, X. Yan, S. Xu, H. Chen, H. Xia and S. Zuo, RSC Adv., 2015, 5, 19900–19906 RSC.
- S. Xu, X. Yan, Q. Bu and H. Xia, RSC Adv., 2016, 6, 8048–8052 RSC.
- C. Fan, H. Guan, H. Zhang, J. Wang, S. Wang and X. Wang, Biomass Bioenergy, 2011, 35, 2659–2665 CrossRef CAS.
- H. Xiong, T. Wang, B. H. Shanks and A. K. Datye, Catal. Lett., 2013, 143, 509–516 CrossRef CAS.
- C. Yue, M. S. Rigutto and E. J. M. Hensen, Catal. Lett., 2014, 144, 2121–2128 CrossRef CAS.
- D. Chen, F. Liang, D. Feng, M. Xian, H. Zhang, H. Liu and F. Du, Chem. Eng. J., 2016, 300, 177–184 CrossRef CAS.
- (a) A. Corma, Chem. Rev., 1995, 95, 559–614 CrossRef CAS; (b) X. Li, Q. Xia, V. C. Nguyen, K. Peng, X. Liu, N. Essayem and Y. Wang, Catal. Sci. Technol., 2016, 6, 7586–7596 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |