Facile synthesis of cationic gold nanoparticles with controlled size and surface plasmon resonance†
Abstract
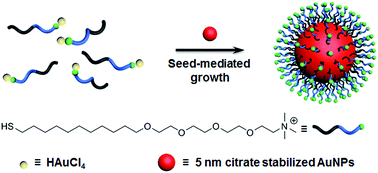
We present a facile synthetic strategy for large cationic gold nanoparticles by utilizing a cationic thiol ligand as a stabilizer for seed-mediated growth. The size and surface plasmon resonance property of the gold nanoparticles were successfully controlled with this strategy.

 Please wait while we load your content...
Please wait while we load your content...