Synthesis of amphiphilic semigrafted pseudo-Pluronics for self-assemblies carrying indomethacin†
Abstract
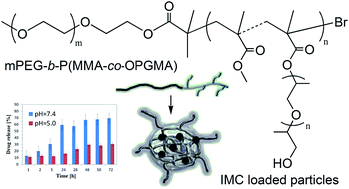
Copolymers with semigrafted topology, consisting of a linear block of poly(ethylene glycol) (PEG) and polymethacrylic segments with loosely distributed oligo(propylene glycol) (OPG) grafts, were obtained by atom transfer radical polymerization (ATRP). The synthesis was performed with the use of a monofunctional macroinitiator (bromoester functionalized PEG) and OPG methacrylate macromonomer (OPGMA) with methyl methacrylate (MMA) comonomer. The amphiphilic copolymers with various compositions of hydrophobic grafted blocks were able to self-assemble in aqueous solution yielding particles with a variety of average sizes (105–210 nm determined by DLS), and the critical aggregation concentration (CAC = 0.032–0.086 mg mL−1 determined by fluorescence spectroscopy). TEM images confirmed the formation of spherical micellar structures. Indomethacin (IMC), a poorly water-soluble drug, was selected as the model drug and encapsulated via the solvent evaporation method with evaluation by drug loading content (DLC = 15–90%). The in vitro release of IMC from polymeric particles in buffer solutions was pH-dependent (lower rates at pH = 5.0 than at pH = 7.4). The results indicated that the linear-b-graft copolymers may be potential carriers for delivery of poorly water-soluble drugs.

 Please wait while we load your content...
Please wait while we load your content...