Nano-curcumin influences blue light photodynamic therapy for restraining glioblastoma stem cells growth†
Abstract
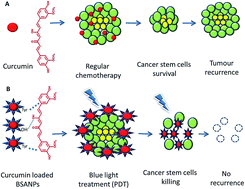
Very low doses of curcumin loaded BSA nanoparticles, following blue light mediated photodynamic therapy, provide improved cytotoxicity against glioblastoma stem cells.

 Please wait while we load your content...
Please wait while we load your content...