Isolation of Amaryllidaceae alkaloids from Nerine bowdenii W. Watson and their biological activities
Abstract
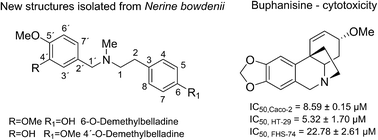
Twenty-two isoquinoline alkaloids (1–22) were isolated from fresh bulbs of Nerine bowdenii (Amaryllidaceae) by standard chromatographic methods. The chemical structures were elucidated by MS, and 1D and 2D NMR spectroscopic analyses, and by comparison with literature data. 6-O-Demethylbelladine (11) and 4′-O-demethylbelladine (12) are reported here for the first time. Compounds isolated in sufficient amounts were evaluated for their acetylcholinesterase, and butyrylcholinesterase inhibition activity using Ellman's method. In the prolyl oligopeptidase assay, Z-Gly-Pro-p-nitroanilide was used as substrate. Untested alkaloids were also screened for their cytotoxic activity against p53-mutated Caco-2 and HT-29 colorectal adenocarcinoma cells. At the same time, healthy small intestine cells FH-74 Int were used to determine overall toxicity against noncancerous cells. The crinine-type alkaloid buphanisine (7) demonstrated interesting cytotoxicity against both tested cancer cell lines with IC50 values of 8.59 ± 0.15 μM for Caco-2 and 5.32 ± 1.70 μM for HT-29.

 Please wait while we load your content...
Please wait while we load your content...