Keratin-reinforced cellulose filaments from ionic liquid solutions†
Kari Kammiovirta,
Anna-Stiina Jääskeläinen,
Lauri Kuutti,
Ulla Holopainen-Mantila,
Arja Paananen,
Anna Suurnäkki and
Hannes Orelma*
VTT, Technical Research Centre of Finland, Biologinkuja 7, FI-02044, Espoo, Finland. E-mail: hannes.orelma@vtt.fi
First published on 12th September 2016
Abstract
Cellulose-based filaments produced with ionic liquid-based processes have high application potential in textiles and composites to replace cotton fibres. These filaments already have unique properties that could be further improved with the addition of proteins. Keratin from poultry feathers is currently a low-value material that has potential as a renewable feedstock in material applications. In this study, cellulose filaments with chicken feather keratin were prepared by wet-spinning from an ionic liquid solution. Both keratin and cellulose were dissolved in [EMIM]AcO and spun into ethanol to regenerate cellulose and keratin and wash out the ionic liquid. The effect of keratin addition on the filament properties was investigated by microscopic, spectroscopic and strength analyses. It was observed that a small keratin addition into the cellulosic filaments improved the mechanical properties remarkably, whereas high keratin additions resulted in reduced mechanical performance. Keratin accumulation on the surface of the prepared filaments was observed. In addition, based on FTIR spectroscopy, it is likely that the morphology of cellulose changed from cellulose I to II and the β-sheets in feather keratin unfolded to unordered keratin upon dissolution and regeneration. The cellulose–protein filaments may find applications from areas where good biocompatibility and easy modifiability are required characteristics.
Introduction
Widespread concern over greenhouse gas emission creates both political and social pressure to find new innovative solutions for the production of energy, fuels, and bio-based products.1,2 Wood-based cellulose is one of the materials that mitigate carbon emissions3 by photosynthetic sugar recovery. Cellulose, the most abundant polymer in nature, has unique inherent properties, such as non-toxicity, mechanical robustness, easy modifiability, hydrophilicity, biocompatibility, and biodegradability.4 The favourable properties of man-made cellulosic fibers have made them an attractive alternative to cotton in industrial and commercial textile products. Moreover, cellulosic filaments may find applications from cellulosic composites where keratin–cellulose filaments could be useful for tailoring matrix–filament interactions. Even if cellulose has been utilized in man-made fibre and filament production for various applications already for decades,5 the production routes are intensively studied to find economically more feasible and more sustainable technologies for their industrial processing. Novel innovations, such as ionic liquid based direct dissolution routes6 provide novel technology to manufacture man-made cellulose filaments with properties and process setups that are superior7,8 to those of the traditional chemical based methods with or without derivations (some examples are viscose, cupra-ammonium, and NMMO).9 Moreover, some ionic liquids have been observed to be capable of solubilizing also enzymes10 and some biopolymers (starch,11 lignin,12 and keratin13), allowing manufacturing of multicomponent filaments with combined functionalities from utilized materials.Keratin is a structural protein found in feathers, human and animal hairs, wool, nails, horns, hooves, and claws.14 These can be considered as waste proteins since they are mostly disposed as landfill, used as low nutritional value animal feed or incinerated. The most abundant keratin source is the poultry feathers,14 which is produced 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons (7.7 × 108 kg) annually as by-product of the food industry around the world.15 Chicken feathers are composed of ca. 90% of keratin, which is tightly packed in β-sheet secondary structure and is insoluble in common organic solvents,16,17 but ionic liquids, such as 1-butyl-3-methylimidazolium chloride (BMIMCl) and 1-allyl-3-methylimidazolium chloride (AMIMCl) have been reported to be capable to its' dissolution.18
000 tons (7.7 × 108 kg) annually as by-product of the food industry around the world.15 Chicken feathers are composed of ca. 90% of keratin, which is tightly packed in β-sheet secondary structure and is insoluble in common organic solvents,16,17 but ionic liquids, such as 1-butyl-3-methylimidazolium chloride (BMIMCl) and 1-allyl-3-methylimidazolium chloride (AMIMCl) have been reported to be capable to its' dissolution.18
The tight packing and intermolecular disulphide bonding of keratin results in some interesting properties such as a resistance against common proteolytic enzymes19 and biological degradation.20 It has been observed that keratin binds metal cations due to the rich thiol group of cysteine composition that promotes its use in filtration membranes.21,22 Proteins, including keratin, have relatively high flame resistance due to high nitrogen content.23 Keratin has also properties promoting biomedical applications24 as it has been shown to promote blood hemostatic by polymerizing fibrinogen to fibrin in the case of injury.25 Moreover, rich thiol composition of keratin can be utilized easily by installing26 functionalities as shown with lyzosymes.27 If these properties could be embedded in cellulosic filaments by adding keratin in them, this would enlarge the possibilities to apply cellulosic filaments in novel applications.
Keratin–cellulose composites have been produced by dissolving cellulose and wool or feathers in ionic liquid and regenerating them in antisolvent.13,28,29 These composites have improved thermal stability of the individual components but the mechanical properties (Young's modulus and tensile strength) were impaired when compared to pure cellulose films.28
Various ionic liquids are able to dissolve keratin.18 However, the dissolution of more ordered cellulose is challenging and only some solvent systems including ionic liquids as 1-ethyl-3-methylimidazolium acetate [EMIM]AcO and 1-butyl-3-methylimidazolium chloride [BMIM]Cl are capable to disrupt the hydrogen bonding between cellulose chains in a cellulose crystallite.30 Therefore, we selected [EMIM]AcO that is reported to be capable to dissolve both keratin and cellulose to be utilized in the filament manufacturing. In this study feather keratin–cellulose composite filaments were prepared by dissolving both cellulose and feather in [EMIM]AcO followed by their wet-spinning in ethanol to regenerate these composite filaments (Scheme 1). It is reported that keratin dissolved in ionic liquids contains a significant water soluble fraction and is immiscible with ethanol supporting the utilization of ethanol or methanol as coagulant in filament spinning.18,31 The effects of added keratin in the structure and properties of prepared filaments were investigated with microscopic, spectroscopic, and mechanical testing methods. The proposed procedure provides methodology to improve cellulose-based filament properties with low-valued keratin addition. Keratin–cellulose composite filaments could be utilised in application areas where functionality and biomedical properties could be beneficial.
 | ||
| Scheme 1 Schematic illustration on the manufacturing keratin reinforced cellulose filaments by ionic liquid dissolution and ethanol coagulation. | ||
Experimental section
Raw materials and reagents
Bleached pine kraft pulp (Metsä Fibre, Finland) was used as cellulose and it was first dried and then grinded with Waring blender. Industrial chicken feathers were used as keratin sources and they were washed with detergent (Fairy, Procter & Gamble, consistency of couple droplets in ca. 0.5 liters of water) and acetone, dried and grinded with Waring blender. 1-Ethyl-3-methylimidazolium acetate ([EMIM]AcO) (Ionic Liquids Technologies GmbH, Heilbronn, Germany), was used to dissolve both cellulose and keratin, and it was used as received. All other chemicals used in this study were laboratory grade.Dissolution of cellulose and keratin into [EMIM]AcO
2 g of wood pulp and 2 g of feathers were dissolved separately (final consistencies both 5 w-%) in 40 g of [EMIM]AcO using a Radley's System Reaction Carousel Station with 100 ml reaction flasks at the constant temperature of 130 °C. The bleached wood pulp was mixed into [EMIM]AcO at a consistency of 5 w-% with magnetic mixing. The solution was stirred until an even dissolution was achieved (ca. 150 min).Filament spinning
The spinning dopes were prepared by mixing keratin solution with cellulose solutions with ratios of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30; 50
30; 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; 20
50; 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80; 10
80; 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 and 0
90 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. Since the polymer (keratin or cellulose) concentrations of both solutions were 5%, the concentration of the dope mix was also 5%.
100. Since the polymer (keratin or cellulose) concentrations of both solutions were 5%, the concentration of the dope mix was also 5%.
Cellulose filaments with and without keratin were prepared by using the wet-jet spinning approach using a laboratory scale spinning system. The spinning dope was placed in a 10 ml plastic syringe, and extruded then through a nozzle (diameter and length of 0.41 μm and 31.75 mm, respectively) into a pure ethanol coagulation bath. The spinning rate was kept constant 0.5 ml min−1. After spinning, the filaments were kept in ethanol until it was clearly opalescent (approximately 30 min) to remove the residual [EMIM]AcO. Then the produced filaments were placed in MilliQ water for two hours, and they were dried under tension at room temperature and ambient humidity.
Distribution of filament components by confocal laser scanning microscopy (CLSM)
The distribution of keratin in the prepared cellulose–keratin filaments was investigated by CLSM and using cellulose and protein specific stains. Prior to embedding, filaments with 90% and 30% were dried in desiccator overnight. Filaments were embedded into hydroxyethyl methylacrylate resin (Leica Historesin embedding kit, Leica Microsystems, Heidelberg, Germany) without further desiccation steps. Polymerized sample blocks were cut in a rotary microtome HM 355S (Microm Laborgeräte GmbH, Walldorf, Germany) using a tungsten carbide knife. Protein and cellulose in the cross-cut surface of the filaments on the sample block were stained with aqueous 0.1% (w/v) acid fuchsin (BDH Chemicals Ltd., Poole, Dorset UK) in 1.0% acetic acid,32 and aqueous 0.01% (w/v) calcofluor white33 (Fluorescent brightener 28, Aldrich, Germany), respectively. The stained surface was imaged using CLSM equipment consisting of a Zeiss LSM 710 (Zeiss, Jena, Germany) attached to a Zeiss Axio Imager.Z microscope. Diode laser line of 405 nm was used for excitation of calcofluor and acid fuchsin and emissions were collected at 410–550 nm and 600–720 nm, respectively. Images were assembled of the optical sections taken using a 20× objective (Zeiss EC Epiplan-Neofluar, numerical aperture of 0.50) to the depth of 10 μm with 2.0 μm z step using ZEN software (Zeiss). The final CLSM micrographs, in which cellulose appear blue and protein red, were reconstructed by superimposing two emission images. Representative images were selected for publication.Elemental analysis
Elemental analysis (C, H, N, S and O) was performed using FLASH 2000 series analyser after drying the sample at 105 °C for overnight to remove any excess moisture. The theoretical elemental composition of cellulose was calculated based to the carbon, nitrogen, and oxygen composition of an anhydroglucose unit (C6H10O6).FTIR-ATR investigations
The chemical composition of the prepared cellulose–keratin filaments were characterized with an FT-IR spectrometer equipped with an ATR diamond crystal (Thermo Scientific Nicolet iS50, United States). Spectra of raw cellulose pulp and grinded chicken feathers were acquired for reference to assess the impact of dissolution and regeneration of the structure of these polymers. Three parallel measurements were collected from different positions of each filament. The filaments were directed in constant angle with respect to the excitation light to avoid any distortion of light polarisation due to the polymer orientation. All spectra were acquired in transmission mode with 32 scans with a spectral range of 4000–400 cm−1 and spectral resolution of 4 cm−1.Scanning electron microscopy (SEM)
The filaments cross-sections were prepared for the imaging by cutting each filament with a surgery knife. The samples were attached on carbon adhesive discs and no conductive coating was applied on the specimen prior SEM imaging. The filaments were imaged with a SEM (LEO DSM 982 Gemini FEG-SEM, Noran Instruments Inc. Middleton, USA) using acceleration voltage of 1.50 keV.Mechanical strength
The mechanical strength of the prepared cellulose–keratin filaments were measured with a tensile tester (a Lloyd LS5 equipped with a 100N sensor, AMETEK Measurement & Calibration Technologies, Florida, USA) under the standard conditions (RH 50% and temperature 23 °C). At least ten different filaments of each sample point were measured. Filament's tensile strengths were normalized with the filament cross-sectional area determined by measuring the filaments thicknesses with a digital micrometer gauge and assuming a circular cross-section. Filament tenacities (textile strengths) were analyzed by first measuring the linear mass–lengths (titer) with a titer tester (Lenzing Vibroskop 400, Lenzing instruments, Gampern, Austria). Then the filament breaking forces were measured with the Lloyed LS5 tensile tester. At least ten identical filaments per each sample point were measured.Results and discussion
Dissolution of cellulose and keratin, preparation of the spinning dope, and preparation of cellulose–keratin filaments
Ionic liquids have been found to be exceptional solvents to disintegrate strong crystalline structures such as wood6 and feathers.18 In this study 1-ethyl-3-methylimidazolium acetate ([EMIM]AcO) was used to solubilize both wood cellulose and feather keratin. However, it is important to note that all ionic liquids that are capable to dissolve both cellulose and keratin are usable with the concept used here. The dissolution was carried out at temperature 130 °C, in which any residual water from wood pulp or feathers evaporates. It was visually observed that cellulose dissolved in [EMIM]AcO in 150 min, after which no visible particles were observed in the solutions (Fig. 1a). At this point, the solution was clear and viscous which is in accordance with earlier studies where cellulose dissolution into [EMIM]AcO and [BMIM]Cl has been investigated.34 The dissolution of pre-treated feathers, containing 90% of keratin,35 which contains 7% cysteine,36 was also carried out at 130 °C temperature to achieve complete dissolution because it is has been observed earlier that lower temperatures than 130 °C may lead to only partial dissolution with [BMIM]Cl.18 We observed that chicken feathers can be dissolved into [EMIM]AcO with concentrations up to 25 w-% after which incomplete dissolution could be achieved. The feather solubility in [EMIM]AcO was thus slightly lower than has been illustrated for [BMIM]Cl, [AMIM]Cl, and choline thioglytate, in which feathers could be dissolved in concentrations up to 50%.18 In the spinning dopes utilized for filament spinning, max 5% feather consistencies were employed where full dissolution was obtained relatively quickly and no visible feather particles were observed. The viscosity of 5% feather solution was lower and the color was darker than that of cellulose (Fig. 1a).The keratin–cellulose spinning dopes were prepared with a constant 5% consistency including both cellulose and keratin in [EMIM]AcO. Dissolved cellulose and keratin were mixed with mixing ratios varying from 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 10
0 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, respectively. The spinning dopes were highly stable as the same solutions could be processed for the filament manufacturing even after several months without affecting the spinnability or deteriorating the filament properties.
90, respectively. The spinning dopes were highly stable as the same solutions could be processed for the filament manufacturing even after several months without affecting the spinnability or deteriorating the filament properties.
The cellulose–keratin filaments were prepared by utilizing wet spinning approach (Fig. 1b) in which the dope containing cellulose and feathers in [EMIM]AcO was extruded directly into an ethanol bath via a nozzle. As soon as the dope contacted ethanol, the filament coagulated and formed the filament structure. The utilization of ionic liquids in cellulose filament productions has been reported in several reports earlier.8,34,37 The spinning setup was kept constant for each spinning dopes to compare the effect of the added keratin in the cellulose filament structure. The cellulose–keratin dopes produced uniform filaments up to 70% keratin consistency (Fig. 1b). However, with dope containing 90% of keratin, no uniform filament was formed and the dope coagulated, typically, into ca. 5 cm long fibres. This result is consistent with literature, since it has been reported that pure keratin filaments have poor mechanical properties.24
The distribution of keratin in the prepared filaments was investigated with CLSM combined with the staining of both keratin and cellulose. In this study, acid fuchsin dye was employed to selectively stain keratin, shown as the red colour in CLSM images, and cellulose was dyed with calcofluor that is a glucan specific stain shown as the blue colour in CLSM images. Fig. 2 shows CLSM images of the cross-sections of filaments with 10 and 70% of keratin (the repetitions can be found from the ESI, images S1 and S2†). It can be seen that with keratin content of 10%, it is quite evenly distributed through the cross-section (see the overlay images). Whereas, when the keratin content was increased to 70%, it enriched on the filament surface, and the inner parts of the filament showed lower keratin content than at the surface. It is conceivable that cellulose can evenly fasten low keratin contents when the cellulose–keratin dope is regenerated into a filament. When the keratin consistency was increased, the regeneration processes of cellulose and keratin separated too much causing uneven solidification when the dope was regenerated by ethanol. It is reported that feather keratin consists of approximately 40% hydrophilic and 60% hydrophobic groups in the amino acid sequence,38 which may also influence to the regeneration process in ethanol via uneven coagulation and leaking out from the filament. Moreover, the filament with the 10% keratin content had a circular cross-section that was disrupted when higher contents than 10% was used.
Chemical analyses of prepared filaments
The composition of the filaments was evaluated based on their elemental composition. Table 1 illustrate that the pure cellulosic filament contained slightly higher proportion of carbon than would be expected for pure cellulosic sample. Based on the molecular formula of anhydroglucopyranose unit (C6H10O6), pure cellulose contains 40% of carbon and 5.6% of hydrogen and the rest is oxygen (54%). However, the filament made from pure cellulosic sample contained higher carbon and hydrogen contents, 44% and 6%, respectively, which indicates remarkably lower oxygen content than for pure cellulose (50%). This could be explained by minor residues of ionic liquids in the filament, which could also explain the nitrogen observed in the cellulosic sample. The nitrogen content of 0.1% on sample weight indicates a residue of 0.6% of ionic liquid on sample weight, which, on the other hand, shows that only minute content of ionic liquid retained in the filaments.| Carbon, % | Hydrogen, % | Nitrogen, % | Sulphur, % | Protein content (%) | |
|---|---|---|---|---|---|
| Cellulose, theoretical | 40.45 | 5.566 | 0 | 0 | 0 |
| 100% cellulose | 44.14 ± 0.06 | 6.28 ± 0.01 | 0.10 ± 0.01 | n.d. | 0.6 |
| 90% cellulose | 44.37 ± 0.01 | 6.30 ± 0.01 | 0.23 ± 0.01 | n.d. | 1.4 |
| 80% cellulose | 45.05 ± 0.12 | 6.41 ± 0.04 | 0.37 ± 0.01 | n.d. | 2.3 |
| 50% cellulose | 45.28 ± 0.79 | 6.40 ± 0.07 | 0.93 ± 0.03 | n.d. | 5.8 |
| 30% cellulose | 44.83 ± 1.87 | 6.36 ± 0.27 | 2.13 ± 0.21 | n.d. | 13.3 |
| 10% cellulose | 48.45 ± 0.08 | 6.82 ± 0.01 | 7.09 ± 0.11 | 0.14 ± 0.02 | 44.3 |
The protein content was determined based on the nitrogen content of the filament by using a conversion factor of 6.25 to calculate the protein content of the sample. The protein contents of the filament samples were surprisingly low, since even at 50% of protein addition the protein content of the filaments was less than 5%. This result is consistent with the CLSM images, where hardly any protein was observed at 10% keratin addition and with 70% keratin addition, the protein consistency was greatest in the filament surface. The low retention of keratin in the filament means that most of the feather added in the system was soluble into ethanol and hence did not coagulate during the filament regeneration.
FTIR-ATR spectroscopy was applied to study the morphological changes in cellulose and keratin upon the dissolution and regeneration. The composition of pure cellulosic pulp before and after dissolution with [EMIM]AcO and regeneration in ethanol into a filament was investigated. The pure cellulosic pulp introduced typical characteristic bands for carbohydrates as illustrated in Fig. 3a. The bands at 1200–850 cm−1 originate from the stretching and bending vibrations of CH, CH2, COH, COC and CO in cellulose molecules, as has been assigned in more details elsewhere.39 Based on FTIR spectroscopy, the cellulose dissolution and regeneration induced a (partial) change in the morphology of cellulose crystalline structure. This can be observed in most details in the peak shifts of the bands at 1022 cm−1 to 1015 cm−1 and 895 cm−1 to 990 cm−1 which correspond to the CO stretching vibrations at C6 position of the anhydropyranose units. In addition, the absorbance of the band at 1337 cm−1 decreased and that of 1263 cm−1 decreased upon cellulose dissolution and regeneration, which are also typical spectral changes as the cellulose morphology is altered from cellulose I to cellulose II.39
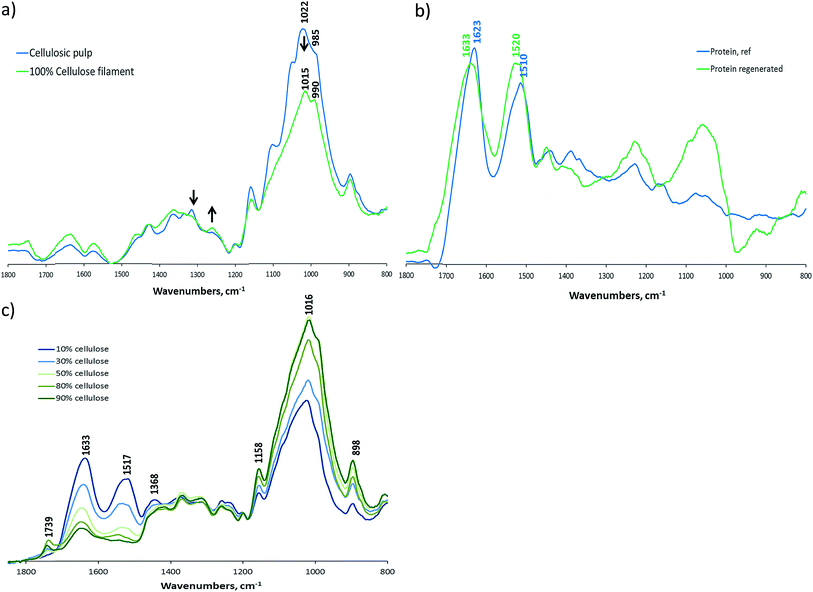 | ||
| Fig. 3 FTIR spectra of (a) cellulose pulp and regenerated cellulose, (b) pure feather and regenerated feather, and (c) cellulose–keratin filaments as a function of cellulose consistency. | ||
The FTIR spectrum of protein reference sample measured from feathers prior to their dissolution was compared to the spectrum of feather protein in the filaments in Fig. 3b. The regenerated protein spectrum was obtained by subtracting the cellulose spectrum from the protein-containing (10% cellulose) filament spectrum. These spectra illustrated that the native feather protein had strongest absorption bands at 1626 cm−1 and 1515 cm−1, whereas the regenerated feather protein had similar bands, but their positions were shifted to 1633 cm−1 and 1520 cm−1.
The bands close to 1650 cm−1 arise mainly from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the amide I and this band is strongly affected by the secondary structure of the backbone but only weakly affected by the nature of side chains.40 The assignment of proteins with different secondary structures has revealed that β-sheets exhibit bands at 1623–1641 cm−1 and 1674–1695 cm−1, whereas unordered protein shows as broad band at ca. 1650 cm−1.40 Based on the FTIR spectra of the chicken feather sample, it is thus likely that the untreated feather protein contained mostly β-sheets, which can also be expected based on literature on feather keratin structure.41,42 The remarkable shift of the amide I band in the FTIR spectrum upon feather dissolution and regeneration indicates that the β-sheets were unfolded and the protein in the filament samples was unordered. This conclusion was thus different than was made for dissolution of chicken feathers in other ionic liquids, where no conclusions on the changes in keratin secondary structure were identified based on FTIR spectroscopy.28,29
O stretching vibration of the amide I and this band is strongly affected by the secondary structure of the backbone but only weakly affected by the nature of side chains.40 The assignment of proteins with different secondary structures has revealed that β-sheets exhibit bands at 1623–1641 cm−1 and 1674–1695 cm−1, whereas unordered protein shows as broad band at ca. 1650 cm−1.40 Based on the FTIR spectra of the chicken feather sample, it is thus likely that the untreated feather protein contained mostly β-sheets, which can also be expected based on literature on feather keratin structure.41,42 The remarkable shift of the amide I band in the FTIR spectrum upon feather dissolution and regeneration indicates that the β-sheets were unfolded and the protein in the filament samples was unordered. This conclusion was thus different than was made for dissolution of chicken feathers in other ionic liquids, where no conclusions on the changes in keratin secondary structure were identified based on FTIR spectroscopy.28,29
The band at ca. 1550 cm−1 arises primarily from the C–N bending vibrations in amide II, which is also mostly affected by the secondary structure of proteins instead of the nature of side chains. However, this range is much more challenging to assign to the changes in protein secondary structure. Therefore it is only concluded that the changes in protein structure upon dissolution and regeneration reflect also a remarkable shift in the amide II absorption band position, although its correlation with the structural changes could not be identified in details.
The FTIR spectra of the filament samples with varying cellulose–protein ratio are illustrated in Fig. 3c. These spectra show basically the increase in the amide I and II bands with respect to cellulose bands with increasing protein content of the filaments, which was expected due to the increase in keratin content in the samples.
Topographical changes
The topography of the prepared cellulose–keratin filaments were imaged with a Scanning Electron Microscopy (SEM) without a conductive coating. Fig. 4a show a cross-section of the cellulose–keratin filament with 20% keratin content. It can be seen that filament was uniform lacking defects and phase separation. The higher magnifications from the cross-sections with keratin contents of 0, 20, and 50% are shown in Fig. 4b–d. Interestingly the cross-section of pure cellulose exhibited small pores, which were disappeared when keratin content increased to 20%. However, at 50% keratin content the small pores in the filament small pores as measured at centre of the cross-section were visible. Moreover, larger pores were present at the surface (Fig. S3†). This result can be compared with the CLSM microscopy images (Fig. 2), where it was observed that keratin concentrated onto the surface of the filaments at high keratin contents. Therefore, it can be speculated that keratin from [EMIM]AcO regenerates in ethanol into the less ordered form than cellulose. When higher keratin consistencies than 20% are used, more porous surface structure are produced. This could be beneficial when looser surface structure may provide a function as in diagnostical applications or drugs delivery.The surface images were recorded from the cellulose–keratin filaments to collect information on their uniformity. It can be seen that all images exhibited grooves on their surfaces, but otherwise the surfaces were uniform (Fig. 5). The reasons for those grooves could be the nozzle geometry and shrinking during regeneration. However, the grooves were highest when the keratin consistency of the filament was 20%, whereas increasing or decreasing keratin content reduced the roughness of the surface. This support the idea that the regeneration based shrinking could be the main reason since to the densest inner structure may cause highest radial forces. It can be seen also from the SEM images that pure cellulose filament surface contained more details, which were randomly oriented, and those were lacking from the filaments with added keratin. This could be explained with the lower viscosity of dissolved keratin compared to equal concentrate cellulose. Then keratin could lubricate the dope, during spinning, leading to lower hydrodynamical forces in the nozzle, which may cause observed details in the filament surface structure.
 | ||
| Fig. 5 Scanning electron microscopy image of the surfaces of the prepared cellulose keratin filaments with keratin consistencies of 0% (a and b), 20% (c and d), and 50% (e and f). | ||
It seems that the compatibility of keratin and cellulose was excellent due to the lacking of phase separation between keratin and cellulose and a rough surface structure. However, in the CLSM studies (shown above) keratin was unevenly distributed in the filament. Moreover, the SEM images evidence complete dissolution of chicken feathers in [EMIM]AcO due to the lack of residual material with large particle size.
Mechanical testing of filament performance
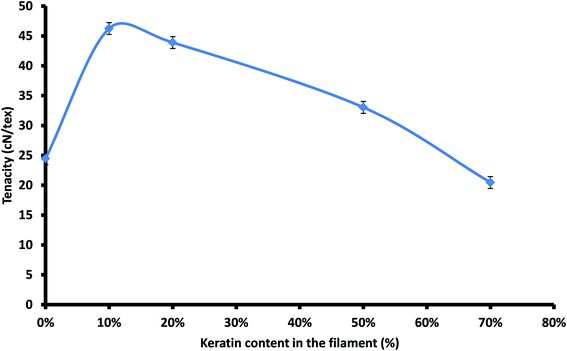
The mechanical performance of the prepared cellulose–keratin filaments were investigated using tensile testing. The tenacity, the ultimate breaking force divided by the linear mass density, of pure cellulose filament was 25.5 ± 0.8 cN per tex (Fig. 6). This value is lower that of reported in the literature for filaments regenerated from [EMIM]AcO (45 cN per tex).34 The difference could be explained by the different spinning technique since dry spinning is known to produce better filament orientation than wet spinning. A better filament orientation can be achieved due to the gravitational stretching at the air-gap and higher orientation will improve the strength properties of a cellulose filament.43Addition of small amount of keratin in the filament increased the filament strength properties remarkably (Fig. 6). The highest filament tenacity (46.2 ± 1.8 cN per tex) was achieved with a 10% keratin addition. Furthermore the tenacity remained above 40 cN per tex at 20% keratin content. However, with higher keratin contents the filament strength reduced. The filaments with keratin addition above 70% could not be analysed due to their poor strength properties.
Other mechanical properties of the filaments as a function of keratin addition are shown in the Table 2. The relatively high thickness of the filaments (206–232 μm) was characteristic to the laboratory spinning setup. Moreover, high standard deviation points out the lack of stretching during the spinning process. The filament thicknesses decreased as a function of added keratin that could be due to the lower viscosity of keratin-containing dopes which enabled easier spinning. It was observed that 5% keratin in [EMIM]AcO was significantly less viscous than the 5% cellulose dope (data observed during the spinning process).
| Cellulose content (%) | Filament thickness (μm) | Titer (tex) | Tensile strength (MPa) | Youngs modulus (GPa) | Stiffness (N m−1) | Elongation (%) |
|---|---|---|---|---|---|---|
| 100 | 232 ± 11 | 21.4 ± 5.6 | 125.4 ± 4.6 | 5.6 ± 0.2 | 5922.6 ± 218.7 | 17.1 ± 4.7 |
| 90 | 220 ± 13 | 11.5 ± 1.5 | 142.4 ± 6.4 | 7.5 ± 0.7 | 7165.5 ± 627.5 | 19.3 ± 1.1 |
| 80 | 222 ± 7 | 12.3 ± 2.7 | 139.86 ± 3.8 | 7.1 ± 0.6 | 6817.4 ± 537.4 | 18.5 ± 2.0 |
| 50 | 206 ± 10 | 13.4 ± 2.9 | 134.58 ± 4.2 | 7.1 ± 0.5 | 5917.1 ± 421.1 | 15.1 ± 0.8 |
| 30 | 221 ± 18 | 15.4 ± 3.8 | 88.4 ± 11.2 | 6.9 ± 0.5 | 6347 ± 467.3 | 9.0 ± 3.7 |
The fineness of a filament expresses the mass–length of the sample and it reflects the density corrected thickness. Addition of 10% of keratin decreased the filament titer from 21.4 to 11.5 tex, respectively. It seems that the rheological behaviour of keratin–cellulose–[EMIM]AcO solution is complex and requires fundamental research to ravel this behaviour.
The elongation of pure cellulose filament was 17%, which was higher compared to the literature value (11%).34 This difference can be explained again with the differences in the preparation processes. The wet-spinning approach leads to lower orientation of cellulose chains than the dry-jet wet spinning. Therefore, cellulose polymers in the filament can elongate more when the prepared filaments are stretched. When 10% keratin was added into the cellulose filament the elongation raised from 17 to 19%, and Young's modulus from 5.6 to 7.5 GPa. This demonstrates that added keratin has positive effect to the filament's strength properties with keratin additions up to 50%.
Conclusions
In this work, cellulose–keratin filaments were investigated. The study revealed that chicken feathers can be effectively dissolved in [EMIM]AcO, which can be employed also a solvent for wood cellulose. The dissolved keratin and cellulose can be easily blended and extruded into a filament with typical wet-spinning method. However, feather protein was only partially coagulated with ethanol and therefore the actual keratin content in the filament was always much lower than the proportion of keratin added in the dope and it was enriched in the filament surfaces. Nevertheless, small addition of keratin had a positive effect on the mechanical properties of cellulose filaments. The investigated method opens new venues for utilizing waste protein materials in cellulosic filaments in future applications. Moreover, the studied approach can be utilized in doping also other proteinic materials than keratin into cellulosic filament if the used IL does not degrade the solubilized protein.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Funding sources
The work was funded by VTT's internal project funding with a government of Finland grant.Acknowledgements
The work was funded by VTT's internal project funding with a government of Finland grant. Unto Tapper is thanked for SEM imaging. Ronny Wahlström is tanked for valuable comments of ionic liquids. Atte Mikkelson is thanked for carrying out elemental analyses. Herbert Sixta is thanked for allowing the use of the titer analyser. Marja Kärkkäinen is thanked for analysing filament titers.References
- B. Schlamadinger and G. Marland, The role of forest and bioenergy strategies in the global carbon cycle, Biomass Bioenergy, 1996, 10(5–6), 275–300 CrossRef CAS.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak and C. L. Liotta, et al., The Path Forward for Biofuels and Biomaterials, Science, 2006, 311(5760), 484–489 CrossRef CAS PubMed.
- R. Sathre and L. Gustavsson, Using wood products to mitigate climate change: external costs and structural change, Appl. Energy, 2009, 86(2), 251–257 CrossRef.
- D. Klemm, Comprehensive cellulose chemistry, fundamentals and analytical methods, Wiley-VCH, Weinheim, 1998, vol. 1 Search PubMed.
- D. Ciechańska, E. Wesołowska and D. Wawro, 1-An introduction to cellulosic fibres A2, in Woodhead Publishing Series in Textiles, ed. S. J. Eichhorn, J. W. S. Hearle, M. Jaffe and T. B. T.-H. Kikutani, Woodhead Publishing, 2009, vol. 2, pp. 3–61 Search PubMed.
- I. Kilpeläinen, H. Xie, A. King, M. Granstrom, S. Heikkinen and D. S. Argyropoulos, Dissolution of Wood in Ionic Liquids, J. Agric. Food Chem., 2007, 55(22), 9142–9148 CrossRef PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, Dissolution of Cellulose with Ionic Liquids, J. Am. Chem. Soc., 2002, 124(18), 4974–4975 CrossRef CAS PubMed.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Dissolution of cellulose with ionic liquids and its application: a mini-review, Green Chem., 2006, 8(4), 325–327 RSC.
- T. Heinze and A. Koschella, Solvents applied in the field of cellulose chemistry: a mini review, Polimeros, 2005, 15(2), 84–90 CAS.
- M. B. Turner, S. K. Spear, J. D. Holbrey and R. D. Rogers, Production of Bioactive Cellulose Films Reconstituted from Ionic Liquids, Biomacromolecules, 2004, 5(4), 1379–1384 CrossRef CAS PubMed.
- K. Wilpiszewska and T. Spychaj, Ionic liquids: media for starch dissolution, plasticization and modification, Carbohydr. Polym., 2011, 86(2), 424–428 CrossRef CAS.
- Y. Pu, N. Jiang and A. J. Ragauskas, Ionic Liquid as a Green Solvent for Lignin, J. Wood Chem. Technol., 2007, 27(1), 23–33 CrossRef CAS.
- H. Xie, S. Li and S. Zhang, Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibers, Green Chem., 2005, 7(8), 606–608 RSC.
- K. Chojnacka, H. Górecka, I. Michalak and H. Górecki, A Review: Valorization of Keratinous Materials, Waste Biomass Valorization, 2011, 2(3), 317–321 CrossRef.
- A. Grazziotin, F. A. Pimentel, E. V. de Jong and A. Brandelli, Nutritional improvement of feather protein by treatment with microbial keratinase, Anim. Feed Sci. Technol., 2006, 126(1–2), 135–144 CrossRef CAS.
- J. A. Maclaren, The extent of reduction of wool proteins by thiols, Aust. J. Chem., 1962, 15(4), 824–831 CrossRef CAS.
- K. Yamauchi, A. Yamauchi, T. Kusunoki, A. Kohda and Y. Konishi, Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films, J. Biomed. Mater. Res., 1996, 31(4), 439–444 CrossRef CAS PubMed.
- A. Idris, R. Vijayaraghavan, U. A. Rana, D. Fredericks, A. F. Patti and D. R. MacFarlane, Dissolution of feather keratin in ionic liquids, Green Chem., 2013, 15(2), 525–534 RSC.
- A. A. Onifade, N. A. Al-Sane, A. A. Al-Musallam and S. Al-Zarban, A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources, Bioresour. Technol., 1998, 66(1), 1–11 CrossRef CAS.
- B. Bálint, Z. Bagi, A. Tóth, G. Rákhely, K. Perei and K. Kovács, Utilization of keratin-containing biowaste to produce biohydrogen, Appl. Microbiol. Biotechnol., 2005, 69(4), 404–410 CrossRef PubMed.
- A. Aluigi, A. Corbellini, F. Rombaldoni and G. Mazzuchetti, Wool-derived keratin nanofiber membranes for dynamic adsorption of heavy-metal ions from aqueous solutions, Text. Res. J., 2013, 83(15), 1574–1586 CrossRef.
- M. T. Cahill, W. D. Anderson, A. R. Elbert, P. B. Perley and R. D. Johnson, Elemental Profiles in Feather Samples from a Mercury-Contaminated Lake in Central California, Arch. Environ. Contam. Toxicol., 1998, 35(1), 75–81 CrossRef PubMed.
- L. Benisek, Flame-Retardant Polymeric Materials, ed. M. Lewin, S. M. Atlas and E. M. Pearce, Springer, Boston, MA, US, 1975, pp. 137–191 Search PubMed.
- J. G. Rouse and M. E. Van Dyke, A Review of Keratin-Based Biomaterials for Biomedical Applications, Materials, 2010, 3(2), 999–1014 CrossRef.
- M. B. Rahmany, R. R. Hantgan and M. Van Dyke, A mechanistic investigation of the effect of keratin-based hemostatic agents on coagulation, Biomaterials, 2013, 34(10), 2492–2500 CrossRef CAS PubMed.
- G. T. Hermanson, Bioconjugate techniques, Academic Press, San Diego, CA, 2008, vol. 2 Search PubMed.
- A. Kurimoto, T. Tanabe, A. Tachibana and K. Yamauchi, Keratin sponge: immobilization of lysozyme, J. Biosci. Bioeng., 2003, 96(3), 307–309 CrossRef CAS PubMed.
- N. Hameed and Q. Guo, Blend films of natural wool and cellulose prepared from an ionic liquid, Cellulose, 2010, 17(4), 803–813 CrossRef CAS.
- R. De Silva, K. Vongsanga, X. Wang and N. Byrne, Development of a novel regenerated cellulose composite material, Carbohydr. Polym., 2015, 121, 382–387 CrossRef CAS PubMed.
- T. Liebert, Cellulose Solvents – Remarkable History, Bright Future, in Cellulose Solvents: For Analysis, Shaping and Chemical Modification; ACS Symposium Series, American Chemical Society, 2010, vol. 1033, pp. 1–3 Search PubMed.
- S. Zheng, Y. Nie, S. Zhang, X. Zhang and L. Wang, Highly Efficient Dissolution of Wool Keratin by Dimethylphosphate Ionic Liquids, ACS Sustainable Chem. Eng., 2015, 3(11), 2925–2932 CrossRef CAS.
- R. G. Fulcher and S. I. Wong, Inside cereals – a fluorescence microchemical view, in Cereals for Food and Beverages, ed. L. B. T.-C. Munck, Academic Press, 1980, pp. 1–26 Search PubMed.
- H. Maeda and N. Ishida, Specificity of Binding of Hexopyranosyl Polysaccharides with Fluorescent Brightener, J. Biochem., 1967, 62(2), 276–278 CAS.
- B. Kosan, C. Michels and F. Meister, Dissolution and forming of cellulose with ionic liquids, Cellulose, 2007, 15(1), 59–66 CrossRef.
- N. Reddy and Y. Yang, Structure and Properties of Chicken Feather Barbs as Natural Protein Fibers, J. Polym. Environ., 2007, 15(2), 81–87 CrossRef CAS.
- R. D. B. Fraser, T. P. MacRae and G. E. Rogers, Keratins: their composition, structure, and biosynthesis, ed. C. C. Thomas, 1972 Search PubMed.
- L. K. J. Hauru, M. Hummel, A. Michud and H. Sixta, Dry jet-wet spinning of strong cellulose filaments from ionic liquid solution, Cellulose, 2014, 21(6), 4471–4481 CrossRef CAS.
- K. M. Arai, R. Takashi, Y. Yokote and K. Akahane, Amino-Acid Sequence of Feather Keratin from Fowl, Eur. J. Biochem., 1983, 132(3), 501–507 CrossRef CAS PubMed.
- S. Y. Oh, D. I. Yoo, Y. Shin and G. Seo, FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide, Carbohydr. Res., 2005, 340(3), 417–428 CrossRef CAS PubMed.
- A. Barth, Infrared spectroscopy of proteins, Biochim. Biophys. Acta, 2007, 1767(9), 1073–1101 CrossRef CAS PubMed.
- R. Schor and S. Krimm, Studies on the Structure of Feather Keratin: II. A β-Helix Model for the Structure of Feather Keratin, Biophys. J., 1961, 1(6), 489–515 CrossRef CAS PubMed.
- R. D. B. Fraser and D. A. D. Parry, Molecular packing in the feather keratin filament, J. Struct. Biol., 2008, 162(1), 1–13 CrossRef CAS PubMed.
- H.-P. Fink, P. Weigel, H. Purz and J. Ganster, Structure formation of regenerated cellulose materials from NMMO-solutions, Prog. Polym. Sci., 2001, 26(9), 1473–1524 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20204g |
| This journal is © The Royal Society of Chemistry 2016 |