Visible light responsive Fe–ZnS/nickel foam photocatalyst with enhanced photocatalytic activity and stability†
Abstract
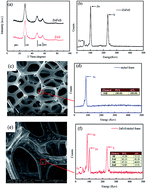
ZnFeS photocatalysts were successfully decorated on nickel foam to form a ZnFeS/nickel foam composite photocatalyst. The ZnFeS/nickel foam not only prevented the photocorrosion-induced release of Zn and Fe ions, but also increased visible light absorption that promoted its charge separation and catalytic activity. The novel photocatalyst displayed a high biphenol A (BPA) removal of 82% within 120 min and excellent stability, which excels other common catalysts for BPA degradation.


 Please wait while we load your content...
Please wait while we load your content...