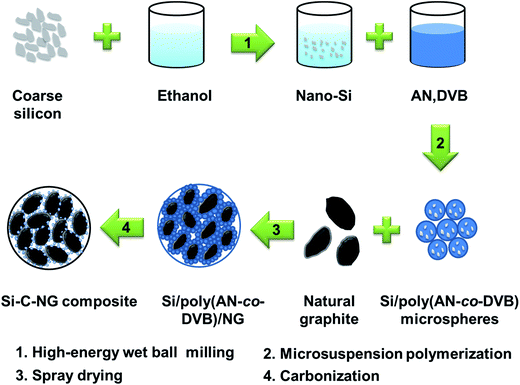
Self-assembly of silicon/carbon hybrids and natural graphite as anode materials for lithium-ion batteries†
Aoning Wang,
Fandong Liu,
Zhoulu Wang and
Xiang Liu*
Key Laboratory of Flexible Electronics (KLOFE), Institue of Advanced Materials (IAM), National Jiangsu Synergistic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (Nanjing Tech), 30 South Puzhu Road, Nanjing, Jiangsu 211816, China. E-mail: iamxliu@njtech.edu.cn
First published on 28th October 2016
Abstract
Silicon/carbon/natural graphite (Si–C–NG) composites have been successfully prepared by the granulation of natural graphite (NG) and silicon/poly(acrylonitrile-co-divinylbenzene) [Si/poly(AN-co-DVB)] hybrid microspheres via spray drying and subsequent pyrolysis. The poly(AN-co-DVB) microspheres containing silicon nano-flakes are synthesized by microsuspension polymerization using benzoyl peroxide (BPO) as initiator and polyvinyl alcohol (PVA) as dispersant in aqueous phase. The morphology and microstructure of Si–C–NG composites are characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive spectroscopy (EDS), X-ray diffraction (XRD) and Raman spectroscopy. It is found that the Si nanoparticles are uniformly enwrapped by pyrolytic carbon derived from poly(AN-co-DVB) to form Si–C core/shell structures, which are subsequently decorated on the surface of NG, resulting in a spherical Si–C–NG structure. When used as anode materials for lithium-ion batteries, the Si–C–NG composites exhibit a high specific capacity of 471.5 mA h g−1, a high initial coulombic efficiency of 78.4%, and a good cycling stability with capacity retention of 87.9% after 100 cycles at a current density of 100 mA g−1.
1. Introduction
Lithium-ion batteries (LIBs), as the most promising power source, have already been widely applied in portable electronics, electric vehicles and energy storage.1–4 Currently, graphite is the most commonly used anode material for LIBs, which shows a good cyclic life and low working voltage. However, the relatively low theoretical capacity (372 mA h g−1) of graphite cannot meet the increasing demand for high energy density, and this therefore restricts its further applications in high-power devices such as electric vehicles. Recently, alternative anode materials for next-generation LIBs, such as silicon (Si), germanium (Ge) and tin (Sn), have been intensively investigated to satisfy the growing demands for large energy density and high power capability. Among the reported materials, Si has attracted remarkable attention due to the highest theoretical capacity (∼4200 mA h g−1) and low stable plateau potential (∼0.4 V).5,6 Unfortunately, the practical application of Si anodes has been restricted mainly by their poor cycling performance due to its extraordinarily large volume expansion (∼300%) during the alloying reaction with lithium ions and low electric conductivity. Such a large volumetric change can lead to pulverization and continuous formation of an unstable solid-electrolyte interphase (SEI) layer, and eventually result in its lower cycling efficiency and rapid capacity fading.7,8 In recent years, many efforts have been made to overcome the aforementioned problems. Some improvements in Si anode performances have been made by constructing Si nanostructures, such as nanoparticles,9,10 nanowires,11,12 and nanotubes,13,14 which can accommodate the mechanical strain caused by volume change and prevent particle cracking. However, nanostructures with a very high specific surface area not only lead to the agglomeration of nano-Si but also cause the risk of excessive side-reactions with the electrolyte.15 To overcome these shortcomings, many strategies have been made, including embedding Si nanoparticles into continuous film structures,13,16 constructing three-dimensional nanoporous Si frameworks,17 encapsulating Si nanoparticles in carbonaceous microspheres,18 and preparing a carbon nanotube or nanofiber/Si composite.19–22 Among all the reported nanostructured Si/C composite materials, the most effective way is to create nanocomposites composed of nano-Si uniformly embedded in the carbon matrix. For instance, Wang et al.23 have successfully synthesized silicon/carbon nanocomposites by dispersing silicon nanoparticles into a gel of phenolic resin followed by carbonization process. The amorphous carbon shell connecting each nano-Si particle, not only has a small volume change and good electrical conductivity, but also protects the silicon from the aggregation during cycling. Recently, Zhang et al.24 prepare the Si-coated modified natural graphite particles by sonicated dispersion and a subsequent heat-treatment process, in which graphite not only serves a buffer for volume change of Si during cycling, but also significantly enhances the cycling stability of Si-based anodes.In addition, enormous efforts have been focused on the preparation of high capacity Si based nanostructured anode materials through morphology and structured control. For example, Liu et al.5 propose a pomegranate-like Si@SiO2@C structured nanocomposite, where each Si nanoparticle is encapsulated by a conductive carbon layer. A reversible capacity of 1160 mA h g−1 at a rate of C/2 has been delivered by Si@SiO2@C nanocomposite. Liu et al.25 report a C@Si@C nanotube sandwich structure which exhibits a reversible specific capacity above 2200 mA h g−1 after 100 cycles. However, it is well known that the mainstream cathode materials in practical applications show relatively low discharge capacities (e.g., LiCoO2 or LiFePO4, 140–170 mA h g−1), which are difficult to fully match with the anode in battery system. Therefore, the requirement for capacity matching of the anode and the cathode should be considered in the design of Si-based composites as the anode.
In this study, considering the capacity matching with the much less energy dense cathode, we proposed a self-assembly Si-based composite anode material with appropriate capacity (around 480 mA h g−1). Herein, nano-Si was encapsulated into a chemically crosslinked poly(AN-co-DVB) hybrid microsphere through microsuspension polymerization, which restrict the agglomeration of nano-Si during cycling and enhance bonding strength between the nano-Si and graphite by means of the conjugated carbon backbones formed by the pyrolysis of poly(AN-co-DVB). Moreover, a new synthetic method for preparation of Si–C–NG composite materials by the granulation of NG and Si/poly(AN-co-DVB) hybrid microspheres via spray drying and subsequent pyrolysis at high temperature, as shown in Scheme 1, is developed. It is found that the as-prepared Si–C–NG composites show a high specific capacity of 471.5 mA h g−1 with an initial coulombic efficiency of 78.41% and an excellent cycling stability with the capacity retention (87.9%) after 100 cycles, compared with Si and NG anodes.
2. Experimental
2.1 Materials
Styrene (St; analytical grade from Aldrich, Shanghai, China), acrylonitrile (AN; analytical grade from Aldrich) and divinylbenzene (DVB; analytical grade from Aldrich, Shanghai, China) were distilled under reduced pressure to remove inhibitors and kept at 0 °C before use. Benzoyl peroxide (BPO; 70%), polyvinyl alcohol (PVA; Mw = 585–146 kDa, degree of hydrolysis 87–89%), polyoxyethylene alkyl ether sulfate, and sodium nitrite (NaNO2; 99%), all from Aldrich, were used as initiator, stabilizer, costabilizer, and inhibitor, respectively, without any further purification. Distilled water was used as the continuous phase. The coupling agent, 3-methacryloxypropyl trimethoxysilane (TMPTS), was purchased from Acros Organics (Acros Organics, Geel, Belgium) and used as received. The chain transfer agent, T-dodecyl mercaptan (TDM) was purchased from Aladdin (Aladdin, Shanghai, China) and used without further treatment. Commercial micrometric Si powder (99.9%, 1–5 micron, Alfa Aesar, Ward Hill, MA, United States) and NG (5–15 μm, Sodiff Advanced Materials Co., Ltd.) were used as raw materials. Sodium carboxymethyl cellulose (CMC; mean Mw = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Acros Organics, Geel, Belgium) was used as binder.
000, Acros Organics, Geel, Belgium) was used as binder.
2.2 Preparation of nano-Si dispersion solution
The preparation of nano-Si dispersion solution was conducted as follows. The coarse silicon powder was mixed with ethanol with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 in a 500 mL holding tank and stirred vigorously for 30 min to form uniform mixture. After that, the mixture was introduced into a ball mill with a zirconia grinding chamber of approximately 160 mL containing 140 mL (apparent volume) zirconia balls (diameter 0.8 mm). The rotating speed was set as 3800 rpm.26
9 in a 500 mL holding tank and stirred vigorously for 30 min to form uniform mixture. After that, the mixture was introduced into a ball mill with a zirconia grinding chamber of approximately 160 mL containing 140 mL (apparent volume) zirconia balls (diameter 0.8 mm). The rotating speed was set as 3800 rpm.26
2.3 Synthesis of Si/poly(AN-co-DVB) hybrid microspheres
Microsuspension polymerization for Si/poly(AN-co-DVB) hybrid microspheres were employed as described previously.27 Firstly, hydrophilic nano-Si was modified before encapsulation: St (25 g), silane coupling agents TMPTS (12.5 g), BPO (1.07 g), and chain transfer agent TDM (1.88 g) were added into a nano-Si dispersion solution (234 g, 10.7 wt%). The mixture was then heated in a 500 mL glass reactor equipped with a reflux condenser under a slow stream of N2 for 12 h at 73 °C to obtain modified nano-Si. Secondly, the modified nano-Si was achieved and the solvent was exchanged from ethanol to monomer mixtures of AN (50 g), DVB (50 g), BPO (4 g), and then they were mixed with 0.67 wt% PVA aqueous solution (450 g) containing polyoxyethylene alkyl ether sulfate (0.02 g), and sodium nitrite (0.3 g). Subsequently, the final mixture was poured into the high-shear dispersing machine (ERS 2000/04, IKNVR Equipment Shanghai Co., Ltd, Shanghai, China) to shear at 9000 rpm for 30 min. Finally, the polymerization was performed at 70 °C for 8 h under a slow stream of nitrogen. The reaction was terminated by cooling the reaction system to room temperature.2.4 Preparation of Si–C–NG composites
The natural graphite (45 g) was first dispersed in 0.5 wt% CMC aqueous solution (55 g) with intense agitation, and followed by the dropping of appropriate proportion of Si/poly (AN-co-DVB) hybrid microspheres with continuous mechanical stirring for 1 h. Subsequently, the obtained suspension was spray dried in a spray dryer unit to form a solid precursor under the following conditions: the rate of suspension delivery was 15 mL min−1; the inlet temperature of the spray dryer was 220 °C; and the outlet temperature was 100 °C. Finally, the as-prepared Si/organic solid precursors were pyrolyzed at 320 °C for 2 h, 450 °C for 2 h and 900 °C for 3 h in a quartz tube furnace under argon atmosphere to obtain the Si–C–NG composites. The resulting composites were ground and sieved for the fabrication of the electrode. For comparison, the C–NG composites without nano-Si were also prepared.2.5 Cell fabrication and electrochemical characterization
Electrochemical measurements were carried out using CR2430 button cells. To prepare working electrodes, the Si–C–NG composites, Super-P carbon black, and poly(vinylidene fluoride) (PVDF, in N-methyl-2-pyrrolidone) with weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were mixed for 6 h to produce a homogeneous slurry and coated onto a 10 μm thick Cu foil. After heating under vacuum at 110 °C for 12 h, the electrode sheet was pressed and punched into 10 mm diameter electrodes. After that, the mass loading and thickness of the active materials was determined to be 1.620 mg cm−2 and 32 μm. The CR2430 button cells were assembled in an argon-filled glove box with lithium metal foil as the counter and reference electrode, Celgard-2400 as the separator and 1 M LiPF6 in a mixture of EC/DEC/EMC (1
10 were mixed for 6 h to produce a homogeneous slurry and coated onto a 10 μm thick Cu foil. After heating under vacuum at 110 °C for 12 h, the electrode sheet was pressed and punched into 10 mm diameter electrodes. After that, the mass loading and thickness of the active materials was determined to be 1.620 mg cm−2 and 32 μm. The CR2430 button cells were assembled in an argon-filled glove box with lithium metal foil as the counter and reference electrode, Celgard-2400 as the separator and 1 M LiPF6 in a mixture of EC/DEC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) as the electrolyte. The charge–discharge performance was tested in a voltage range of 0.01–2.00 V at a constant current of 100 mA g−1 at room temperature using a battery test system (LAND CT2001A model, Wuhan LAND Electronics. Ltd.).
1 by volume) as the electrolyte. The charge–discharge performance was tested in a voltage range of 0.01–2.00 V at a constant current of 100 mA g−1 at room temperature using a battery test system (LAND CT2001A model, Wuhan LAND Electronics. Ltd.).
2.6 Characterization
Scanning electron microscopy (SEM, JEOL, JSM-6360LV) equipped with energy-dispersive X-ray spectroscopy (EDS, EDX-GENESIS) was used to analyze the composition and elemental distribution of samples. The transmission electron microscopy (TEM; Hitachi, HT7700) images were obtained with an acceleration voltage of 100 kV. Powder X-ray diffraction (XRD) was performed on Rint-2000 (Rigaku) diffractometer with Bragg–Brentano θ–2θ geometry (40 kV and 30 mA), using graphite monochromatized Cu Kα radiation. Raman measurement was performed with a Horiba Jobin Yvon Raman spectrometer at the excitation wavelength of 514.4 nm. Thermogravimetric analysis (TGA) was carried out on Mettler-Toledo TGA2 under an oxygen atmosphere from room temperature to 1000 °C at a heating rate of 10 °C min−1. The electrical conductivity of Si–C–NG composites was determined by a powder resistivity measurement system (Loresta).3. Results and discussion
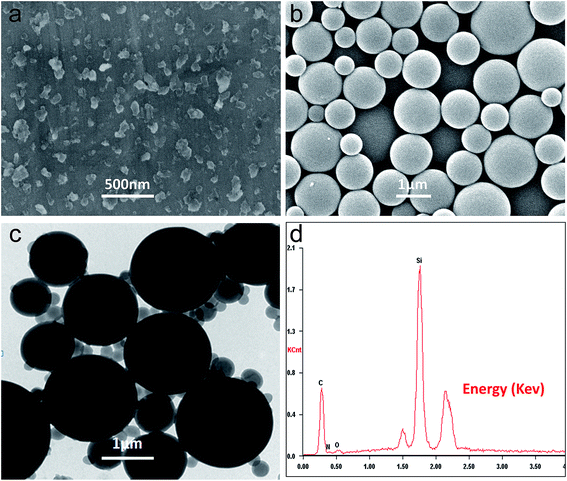
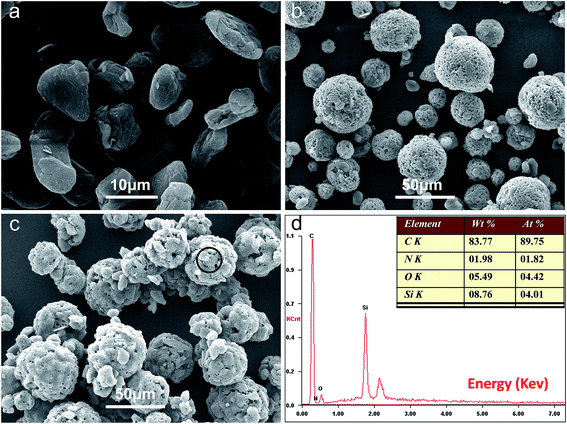
Fig. 1 shows the SEM image of nano-Si, and the SEM, TEM, EDS of Si/poly(AN-co-DVB) hybrid microspheres. As it is illustrated in Fig. 1a, the commercial micrometric silicon powder has been fractured into irregular silicon nano-flakes of about 60–150 nm by the high-energy wet ball milling, and somewhat aggregated due to the high surface energy of nanoparticles. From the SEM image of Si/poly(AN-co-DVB) hybrid microspheres, it displays a relatively smooth spherical shape with a large size distribution, and no nano-Si exposed on the surface of these microspheres could be observed. The EDS of the Si/poly(AN-co-DVB) is shown in Fig. 1d. The presence of a strong Si characteristic peak and the regular microsphere structure of the composite from SEM observation demonstrate that the nano-Si has been introduced inside the polymer microspheres. From the TEM image presented in Fig. 1c, it could be confirmed that the Si/poly(AN-co-DVB) hybrid microspheres are comparatively smooth and spherical in shape with a broad size distribution. The randomly distributed irregular darker areas inside the spheres are believed to be nano-Si. Therefore, it is feasible to prepare the composite microspheres with uniformly distributed nano-Si encapsulated in polymer by the microsuspension polymerization.The SEM images of the NG, Si/poly(AN-co-DVB)/NG and Si–C–NG composites are displayed in Fig. 2. According to the SEM image of the NG presented in Fig. 2a, the layered graphite with relatively sharp boundaries of approximately 10 μm could be observed. As it is shown in Fig. 2b, the Si/poly(AN-co-DVB)/NG particles assembled with NG by spray drying exhibit a waxberry-like morphology and spherical shape with a large size distribution. The majority of NG is covered with Si/poly(AN-co-DVB) hybrid microspheres, suggesting that polymer microspheres play a role of binder in constructing the special spherical structure. The carbonized Si–C–NG composites (Fig. 2c) maintained the spherical shape, but a rough and gapped surface could be observed due to the carbonization of poly(AN-co-DVB). In order to verify the elements in the resulting Si–C–NG composites, EDS pattern was obtained from the shell part of the sample (the circle area of Fig. 2c). A high carbon peak and Si peak could be observed, and the element proportion is listed in the inset of Fig. 2d. It is clear that the Si content and the carbon content in the composites are about 8.76 wt% and 83.77 wt%, which are in well agreement with the designed result. TGA was used to confirm the composition of Si–C–NG composites after the process of carbonization (Fig. S1†). When heated under oxygen atmosphere, the amorphous carbon and NG in the Si–C–NG composites were combusted (weight loss), while the Si in the composites was oxidized to silica (weight gain). When the temperature between 400 and 600 °C, a constant weight loss was found that was ascribed to the combustion of amorphous carbon. The weight loss occurred between 600 and 850 °C was ascribed to the combustion of NG. From the TGA curve, the weight percentage of pyrolytic carbon, NG and Si could be calculated to be approximately 18%, 72%, and 10%, respectively.
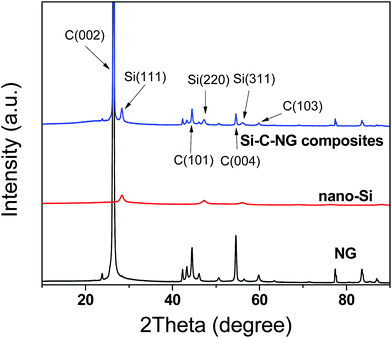
The X-ray diffraction (XRD) patterns of nano-Si, NG and Si–C–NG composites are shown in Fig. 3. The peaks at 26.5°, 44.5°, 54.7° and 59.7° of the NG are corresponding to the lattice planes of (002), (101), (004) and (103).28,29 The XRD of the nano-Si located at 28.4°, 47.3°, 56.1°, 69.1° and 76.4° can be indexed to its lattice planes of (111), (220), (311), (400), and (331), respectively. It is obvious that the XRD pattern of the as-prepared Si–C–NG composites reveals the presence of graphite and Si due to existence of diffraction peaks of graphite and diffraction peaks of Si at 26.5°, 44.5°, 54.7°, 28.4°, 47.3° and 56.1°, respectively. It is suggested that graphite and Si still retain their own special crystalline structure through polymerization, spray drying and subsequent pyrolysis process. Except for the above obvious diffraction peaks, a broad peak centered at approximately 23° which also overlapped with the diffraction peaks of graphite at 26.5° and the diffraction peak of Si at 28.4° was also observed. It is the characteristic diffraction peak of amorphous carbon, which most probably resulted from the pyrolysis of Si/poly(AN-co-DVB) composites. Beyond that, no peaks corresponding to the most possible impurity phase like Si carbide could be detected by the XRD, which confirms that the as-prepared Si–C–NG composites are composed of Si, NG and amorphous carbon derived from poly(AN-co-DVB).
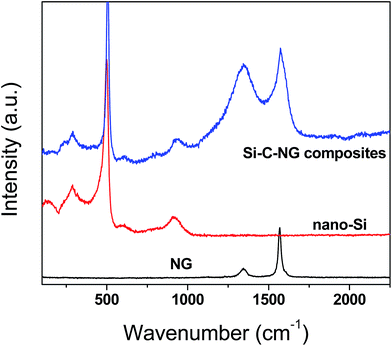
Fig. 4 presents the Raman spectra of nano-Si, NG and Si–C–NG composites. For nano-Si, a strong peak at 515 cm−1 and two broad bands at about 290 cm−1 and around 920 cm−1 corresponding to the Si–Si stretching vibration mode of crystalline Si phase are observed. For NG, the characteristic peaks at 1342 cm−1 and 1568 cm−1 belong to D band and G band. The D-band usually results from structural defects or disorder of carbon while the G-band can be accounted for presence of the stretching vibration mode of crystalline graphite.30 R (ID/IG), the relative peak integrated intensity ratio of the D-band to the G-band, reflects the graphitization degree. The R value greatly affects graphitization degree of the carbon: the lower R value, the higher degree of graphitization. From Fig. 4, the R value of natural graphite was estimated to be 0.42, suggesting a high degree of graphitization. For the as-prepared Si–C–NG composites, the adsorption bands can be assigned to elemental Si at 515 cm−1, 290 cm−1 and 920 cm−1, and another two sharp bands are attributed to the carbon phase at 1342 cm−1 and 1568 cm−1 are all observed in the composites. Moreover, compared to NG, the intensity of the D band in the composites is enhanced and the intensity ratio R is increased to 1.45. In other words, it can be concluded that the amount of disorder of the carbon in the composites was increased in contrast to NG, which is obviously the incorporation of the carbon pyrolyzed from poly(AN-co-DVB). These results confirm that the as-prepared composites from the spray drying/pyrolysis process are the mixture of nano-Si, the NG and amorphous carbon, which are consistent with the XRD analysis.
The powder electrical conductivity of Si–C–NG composites was measured as a function of pressure. The results from the powder resistivity measurement system show that the electrical conductivity of Si–C–NG composites is higher than that of nano-Si and NG mixtures (Fig. S2†). The improved electrical conductivity implies faster electron transfer of Si–C–NG composites, while the continuous pyrolytic carbon plays an important role, which leads to a good contact between the particles and facilitates the charge transfer.31
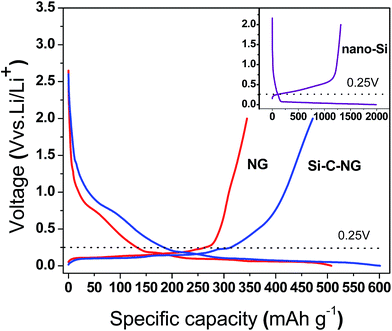
Fig. 5 presents the first charge/discharge profiles of Si–C–NG composites, pure NG and nano-Si at a current density of 100 mA g−1. For the discharge process of all the samples, obvious parallel voltage platform of all curves (including the inset one) at 0–0.2 V can be observed, which is corresponding to the insertion of lithium ions into graphite and/or alloying with the nano-Si. For the charge process of NG and Si–C–NG composites, an obvious voltage plateau of both curves at 0–0.25 V is observed, which is attributed to lithium ions extraction from graphite. However, the charge curve of the Si–C–NG composites shows an extra platform at 0.45 V resulting from the dealloying process of lithium ions with Si,32,33 which is consistent with potential plateau of the charge process of nano-Si in the inset of Fig. 5. Due to the presence of nano-Si and pyrolytic carbon, the Si–C–NG composites electrode delivered a capacity of 1.5 times higher than that of NG, without significantly shift up the charging platform. As seen in Fig. S3,† the capacity contribution of pyrolytic carbon is around 170 mA h g−1. Different from Si and Si–C–NG composites, the pyrolytic carbon presents typical Li-ion insertion/extraction behavior of pyrolytic carbon with a low crystallinity, that is to say, no obvious charge–discharge plateau observed in charge–discharge processes.
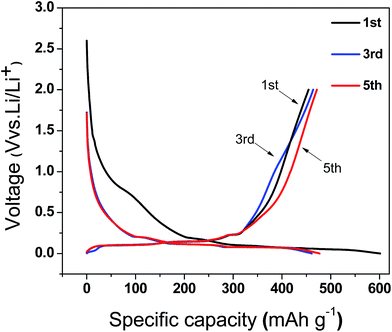
The charge/discharge curves of the Si–C–NG composites electrode at different cycle numbers are illustrated in Fig. 6. The discharge plateau at 0.6–0.9 V of the first cycle can be observed and it is due to the formation of SEI layer on the surface of electrode. This discharge plateau disappears at subsequent cycles and the irreversible capacity decreases correspondingly. The curve shape of the 3rd cycle and those afterwards are identical indicating the good cycle performance of Si–C–NG composites electrode.
Fig. 7 shows the cycle performance of Si–C–NG composites electrode, nano-Si and NG at a constant current of 100 mA g−1. Compared with the NG, the as-fabricated Si–C–NG composites delivered a capacity of 471.5 mA h g−1, which is higher than that of NG (344.5 mA h g−1). Compared to nano-Si, the as-fabricated Si–C–NG composites as an anode maintained an excellent cycling stability. It is worth to note that the capacity of Si–C–NG composites increased gradually before 35th cycle which can be ascribed to the surface activation process of Si nanoparticles. The Si–C–NG composites based batteries exhibit a high coulombic efficiency of above 96% after the first cycle. The high specific capacity of Si–C–NG composites is attributed to the introduction of nano-Si.
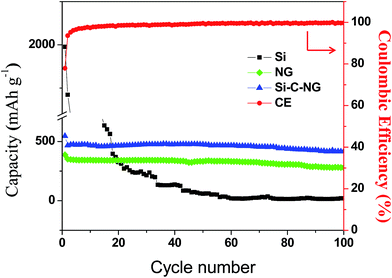 | ||
| Fig. 7 The cycle performances of anodes prepared with nano-Si, NG and the Si–C–NG composites at a constant current of 100 mA g−1 for 100 cycles. | ||
Fig. S4† shows the rate performance of Si–C–NG composites electrode at various current densities. It can be seen that the specific capacities gradually decrease upon increasing the current density from 100 to 1000 mA g−1. The Si–C–NG electrode shows a capacity of ∼470 mA h g−1 at 100 mA g−1 and it changes to 430, 383, 338, 275, and 200 mA h g−1 when the current density increases to 200, 400, 600, 800, and 1000 mA g−1. Notably, the specific capacity is restored to approximately 424 mA h g−1 when the current density returns to 100 mA g−1, indicating a good rate performance of the as-prepared Si–C–NG composites electrode. This excellent rate performance may result from the good electrical and Li+ conductivity of the amorphous carbon pyrolyzed from polymer.
For further identify the structural stability of this composite, the SEM analysis of the Si–C–NG composites electrodes after 30 and 50 cycles were performed (Fig. S5†). It can be seen that the Si–C–NG composites electrodes exhibit a small volume expansion after 30 and 50 cycles and no obvious microcracks can be noticed. Therefore, it is proved that the amorphous carbon pyrolyzed from polymer can buffer the volume change of Si during charging/discharging.
The electrochemical performances of nano-Si, NG and Si–C–NG composites are summarized in Table 1. For nano-Si, the first charge and discharge capacity at 100 mA g−1 are 947.1 mA h g−1 and 1988.3 mA h g−1, the initial coulombic efficiency (ICE) is only 47.63%, and it shows poor capacity retention (1.5% at 100th cycle) due to the high volumetric variation during alloying/dealloying with lithium ions. For NG, it exhibits typical cyclic properties with a relatively low first charge capacity (344.5 mA h g−1), discharge capacity (507.1 mA h g−1) and ICE (67.94%) while it shows excellent capacity retention (88.2% at 100th cycle). The Si–C–NG composites electrode has a relatively high charge (471.5 mA h g−1) and discharge capacity (601.3 mA h g−1) with an initial coulombic efficiency of 78.41% and the capacity retention (87.9%) after 100 cycles. The excellent electrochemical performance, including high specific capacity, good cycle life and high ICE of Si–C–NG composites than pure Si and graphite can be ascribed to its superiority of NG and Si nanoparticles based hierarchical structure. First, the pyrolytic carbon wrapped Si nanoparticles in microspheres not only provides sufficient buffer for volume change of Si during charging/discharging, but also serves as a stable contact between the active material and the current collector. Second, the addition of NG makes the electrode structure more stable during charge/discharge process. Third, the addition of the amorphous carbon pyrolyzed from polymer acts as the binder to combine nano-Si with NG for maintaining composite integrity. The unexpectedly high ICE of Si–C–NG composite may be explained by the introduction of amorphous carbon pyrolyzed from poly(AN-co-DVB) which decreases the active sites of graphite in the composites where the electrolyte decomposes, thus reduces the side reaction and irreversible capacity. All in all, the excellent electrochemical performance of the Si–C–NG composite electrode indicates that the huge volume change of nano-Si during charge–discharge process has been effectively alleviated by the introduction of amorphous carbon and NG.
| Sample | 1st charge capacity (mA h g−1) | 1st discharge capacity (mA h g−1) | ICE (%) | Retention (%) @100th cycle |
|---|---|---|---|---|
| Nano-Si | 947.1 | 1988.3 | 47.63 | 1.5 |
| NG | 344.5 | 507.1 | 67.94 | 88.2 |
| Si–C–NG | 471.5 | 601.3 | 78.41 | 87.9 |
4. Conclusions
Si–C–NG composites have been successfully synthesized, in which nano-Si was encapsulated in crosslinked copolymer microspheres by microsuspension polymerization of AN and DVB at first, and then assembled with NG based on spray drying and subsequent pyrolysis process. From SEM and TEM analysis, homogeneously-dispersed nano-Si is observed in polymer microspheres and the Si–C–NG composites in which nano-Si is uniformly enwrapped by pyrolytic carbon derived from poly(AN-co-DVB) and then covered on the surface of NG exhibit spherical structure but with rough and gapped surface. From XRD analysis, the as-prepared Si–C–NG composites are proved to be a physical mixture of crystalline Si, crystalline graphite and amorphous carbon without any other impurities. From Raman analysis, it can be concluded that the amount of disorder of the carbon in the Si–C–NG composites were increased in contrast to natural graphite. Moreover, compared with Si and NG anodes, the as-prepared Si–C–NG composites show a higher specific capacity of 471.5 mA h g−1 with an initial coulombic efficiency of 78.41% and an excellent cycling stability with the capacity retention (87.9%) after 100 cycles. Considering the relatively low Si content of composites, which is desirable for the specific capacity, this work provides an effective approach to develop high stability and cathode matched Si-based anode materials for practical applications in lithium-ion batteries.References
- Z. Favors, H. H. Bay, Z. Mutlu, K. Ahmed, R. Ionescu, R. Ye, M. Ozkan and C. S. Ozkan, Towards scalable binderless electrodes: carbon coated silicon nanofiber paper via Mg reduction of electrospun SiO2 nanofibers, Sci. Rep., 2015, 5, 8246 CrossRef CAS PubMed.
- M. Ge, J. Rong, X. Fang and C. Zhou, Porous doped silicon nanowires for lithium ion battery anode with long cycle life, Nano Lett., 2012, 12(5), 2318–2323 CrossRef CAS PubMed.
- M. He, Q. Sa, G. Liu and Y. Wang, Caramel popcorn shaped silicon particle with carbon coating as a high performance anode material for Li-ion batteries, ACS Appl. Mater. Interfaces, 2013, 5(21), 11152–11158 CAS.
- D. S. Jung, T. H. Hwang, S. B. Park and J. W. Choi, Spray drying method for large-scale and high-performance silicon negative electrodes in Li-ion batteries, Nano Lett., 2013, 13(5), 2092–2097 CrossRef CAS PubMed.
- N. Liu, Z. Lu, J. Zhao, M. T. McDowell, H. W. Lee, W. Zhao and Y. Cui, A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes, Nat. Nanotechnol., 2014, 9(3), 187–192 CrossRef CAS PubMed.
- F. Zhang, X. Yang, Y. Xie, N. Yi, Y. Huang and Y. Chen, Pyrolytic carbon-coated Si nanoparticles on elastic graphene framework as anode materials for high-performance lithium-ion batteries, Carbon, 2015, 82, 161–167 CrossRef CAS.
- Y. S. Yoon, S. H. Jee, S. H. Lee and S. C. Nam, Nano Si-coated graphite composite anode synthesized by semi-mass production ball milling for lithium secondary batteries, Surf. Coat. Technol., 2011, 206(2–3), 553–558 CrossRef CAS.
- X. Y. Zhou, J. J. Tang, J. Yang, J. Xie and L. L. Ma, Silicon@carbon hollow core–shell heterostructures novel anode materials for lithium ion batteries, Electrochim. Acta, 2013, 87, 663–668 CrossRef CAS.
- X. Chen, X. Li, F. Ding, W. Xu, J. Xiao, Y. Cao, P. Meduri, J. Liu, G. L. Graff and J. G. Zhang, Conductive rigid skeleton supported silicon as high-performance Li-ion battery anodes, Nano Lett., 2012, 12(8), 4124–4130 CrossRef CAS PubMed.
- Z. Luo, Q. Xiao, G. Lei, Z. Li and C. Tang, Si nanoparticles/graphene composite membrane for high performance silicon anode in lithium ion batteries, Carbon, 2016, 98, 373–380 CrossRef CAS.
- S. H. Baek, J. S. Park, Y. M. Jeong and J. H. Kim, Facile synthesis of Ag-coated silicon nanowires as anode materials for high-performance rechargeable lithium battery, J. Alloys Compd., 2016, 660, 387–391 CrossRef CAS.
- H. Wang, H. Song, Z. Lin, X. Jiang, X. Zhang, L. Yu, J. Xu, L. Pan, J. Wang, M. Zheng, Y. Shi and K. Chen, Highly cross-linked Cu/a-Si core–shell nanowires for ultra-long cycle life and high rate lithium batteries, Nanoscale, 2016, 8(5), 2613–2619 RSC.
- J. Chen, L. Bie, J. Sun and F. Xu, Enhanced electrochemical performances of silicon nanotube bundles anode coated with graphene layers, Mater. Res. Bull., 2016, 73, 394–400 CrossRef CAS.
- L. Xiao, Y. H. Sehlleier, S. Dobrowolny, H. Orthner, F. Mahlendorf, A. Heinzel, C. Schulz and H. Wiggers, Si-CNT/rGO Nanoheterostructures as High-Performance Lithium-Ion-Battery Anodes, ChemElectroChem, 2015, 2(12), 1983–1990 CrossRef CAS.
- Y. Yin, L. Wan and Y. Guo, Silicon-based nanomaterials for lithium-ion batteries, Chin. Sci. Bull., 2012, 57(32), 4104–4110 CrossRef CAS.
- L. B. Chen, H. C. Yu, C. M. Xu and T. H. Wang, Investigation of Si film anode prepared by ion beam assisted deposition, Acta. Phys. Sin. Ch. Ed., 2009, 58(7), 5029–5034 CAS.
- P. Gao, H. Jia, J. Yang, Y. Nuli, J. Wang and J. Chen, Three-dimensional porous silicon-MWNT heterostructure with superior lithium storage performance, Phys. Chem. Chem. Phys., 2011, 13(45), 20108–20111 RSC.
- J. Bae, Fabrication of carbon microcapsules containing silicon nanoparticles for anode in lithium ion battery, Colloid Polym. Sci., 2011, 289(11), 1233–1241 CAS.
- Y. Chen, N. Du, H. Zhang and D. Yang, Facile synthesis of uniform MWCNT@Si nanocomposites as high-performance anode materials for lithium-ion batteries, J. Alloys Compd., 2015, 622, 966–972 CrossRef CAS.
- J. Y. Eom and H. S. Kwon, Preparation of single-walled carbon nanotube/silicon composites and their lithium storage properties, ACS Appl. Mater. Interfaces, 2011, 3(4), 1015–1021 CAS.
- C. M. Wang, X. Li, Z. Wang, W. Xu, J. Liu, F. Gao, L. Kovarik, J. G. Zhang, J. Howe, D. J. Burton, Z. Liu, X. Xiao, S. Thevuthasan and D. R. Baer, In situ TEM investigation of congruent phase transition and structural evolution of nanostructured silicon/carbon anode for lithium ion batteries, Nano Lett., 2012, 12(3), 1624–1632 CrossRef CAS PubMed.
- Y. Li, B. Guo, L. Ji, Z. Lin, G. Xu, Y. Liang, S. Zhang, O. Toprakci, Y. Hu, M. Alcoutlabi and X. Zhang, Structure control and performance improvement of carbon nanofibers containing a dispersion of silicon nanoparticles for energy storage, Carbon, 2013, 51, 185–194 CrossRef CAS.
- M. S. Wang and L. Z. Fan, Silicon/carbon nanocomposite pyrolyzed from phenolic resin as anode materials for lithium-ion batteries, J. Power Sources, 2013, 244, 570–574 CrossRef CAS.
- T. Zhang, J. Gao, L. J. Fu, L. C. Yang, Y. P. Wu and H. Q. Wu, Natural graphite coated by Si nanoparticles as anode materials for lithium ion batteries, J. Mater. Chem., 2007, 17(13), 1321 RSC.
- J. Liu, N. Li, M. D. Goodman, H. G. Zhang, E. S. Epstein, B. Huang, Z. Pan, J. Kim, J. H. Choi, X. Huang, J. Liu, K. J. Hsia, S. J. Dillon and P. V. Braun, Mechanically and Chemically Robust Sandwich-Structured C@Si@C Nanotube Array Li-Ion Battery Anodes, ACS Nano, 2015, 9(2), 1985–1994 CrossRef CAS PubMed.
- M. Zhang, X. Hou, J. Wang, M. Li, S. Hu, Z. Shao and X. Liu, Interweaved Si@C/CNTs&CNFs composites as anode materials for Li-ion batteries, J. Alloys Compd., 2014, 588, 206–211 CrossRef CAS.
- F. Liu, Z. Wang, Y. Zhou and X. Liu, Preparation of hybrid composite microspheres containing nanosilicon via microsuspension polymerization, J. Appl. Polym. Sci., 2016, 133(12), 43101 CrossRef.
- Y. Zhou, Z. Tian, R. Fan, S. Zhao, R. Zhou, H. Guo and Z. Wang, Scalable synthesis of Si/SiO2@C composite from micro-silica particles for high performance lithium battery anodes, Powder Technol., 2015, 284, 365–370 CrossRef CAS.
- R. Zhou, R. Fan, Z. Tian, Y. Zhou, H. Guo, L. Kou and D. Zhang, Preparation and characterization of core–shell structure Si/C composite with multiple carbon phases as anode materials for lithium ion batteries, J. Alloys Compd., 2016, 658, 91–97 CrossRef CAS.
- J. B. Koo, B. Y. Jang and K. S. Han, Si–C composites synthesized by using Si nanoparticles and carboxymethyl cellulose as anode materials for lithium-ion batteries, J. Korean Chem. Soc., 2015, 67(10), 1831–1837 CAS.
- S. J. Kim, J. Lee, B. H. Kim, Y. J. Kim, K. S. Yang and M. S. Park, Facile synthesis of carbon-coated silicon/graphite spherical composites for high-performance lithium-ion batteries, ACS Appl. Mater. Interfaces, 2016, 8, 12109–12117 CAS.
- M. Su, Z. Wang, H. Guo, X. Li, S. Huang and L. Gan, Silicon, flake graphite and phenolic resin-pyrolyzed carbon based Si/C composites as anode material for lithium-ion batteries, Adv. Powder Technol., 2013, 24(6), 921–925 CrossRef CAS.
- J. Lai, H. Guo, Z. Wang, X. Li, X. Zhang, F. Wu and P. Yue, Preparation and characterization of flake graphite/silicon/carbon spherical composite as anode materials for lithium-ion batteries, J. Alloys Compd., 2012, 530, 30–35 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20131h |
| This journal is © The Royal Society of Chemistry 2016 |