Durable antifouling polyvinylidene fluoride membrane via surface zwitterionicalization mediated by an amphiphilic copolymer†
Mengyuan Shi,
Jing Zhu and
Chunju He*
The State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620, P. R. China. E-mail: chunjuhe@dhu.edu.cn; Fax: +86-21-67792855; Tel: +86-21-67792842
First published on 28th November 2016
Abstract
The antifouling properties of a polyvinylidene fluoride (PVDF) membrane were remarkably enhanced by facile incorporation of an amphiphilic triblock copolymer poly(N,N-dimethylamino-2-ethylmethacrylate)-b-polydimethylsiloxane-b-poly(N,N-dimethylamino-2-ethylmethacrylate) (PDMAEMA-b-PDMS-b-PDMAEMA) synthesized via the atom transfer radical polymerization and subsequent surface zwitterionicalization, which provided a stable fouling-resistance interface to avoid undesirable “hydrophobic recovery”. As compared with the pure PVDF membrane, the total flux decline ratio of protein pollution parameters is substantially reduced as low as 14.5% and the BSA rejection of the blend membranes is increased from 33% to 90%, which proves the decrease of effective membrane pore size. The dramatic performance enhancement is attributed to the formation of unique rod-like micelles on the zwitterionic membrane surface due to the self-assembly of the amphiphilic polymer during quaternization. The spontaneous migration of PDMAEMA segments to the membrane surface as conformed by XPS measurement results in significant improvement of the wetting properties, and in situ quaternization further expands the migration rate. The continual migration of the internal amphiphilic copolymers can form a renewable surface to ensure excellent antifouling and durability as conformed by the time-dependent flux test for 30 days. Furthermore, the elastic hydrophobic segments PDMS act as anchors in the matrix, which effectively prevent the elution of amphiphilic polymer. This one-step approach to achieve surface zwitterionicalization is promising to provide a simple strategy for the preparation of multifunctional zwitterionic polymers on the surface of polymeric membrane materials to meet with various demands for further applications, i.e. antifouling and antibacterial properties, etc.
1. Introduction
Polyvinylidene fluoride (PVDF) is one of the most promising membrane materials due to its outstanding chemical resistance, thermal stability and mechanical strength as well as good processability.1 However, the strong hydrophobic characteristic of PVDF leads to membrane fouling, resulting in the serious decrease in water flux.2–6 Various techniques,7,8 i.e. chemical grafting,9–13 plasma treatment,14,15 surface coating16–21 and physical blending,22,23 have been carried out to improve the antifouling ability of PVDF membranes. However, chemical grafting is complicated and time-consuming, plasma treatment can change the mechanical properties of the bulk matrix and the instability and pore blocking of the surface coating make it difficult to maintain long-term efficiency. Furthermore, the blending additives, i.e. water-soluble polymers and nanometer particles24–26 are easily washed out from membrane matrix. Recently, the addition of amphiphilic copolymers has been proved to be a facile and versatile strategy with outstanding stable modification effects27–30 since the hydrophobic segments act as anchors in the matrix to hinder the elution of the copolymers from the matrix while the hydrophilic part preferentially segregates to membrane surface and pore walls during the coagulation process.Zwitterionic polymers31–37 have strong affinity with water molecules to avoid foulants absorption and lead to excellent anti-fouling and biocompatibility and have been used in surface modification.38–40 The resulted membranes showed wetting and antifouling properties and excellent hemocompatibility.36,39 However, most literatures have been concerned about the surface grafting of zwitterionic polymer chains on membrane surface due to the low dissolubility of zwitterionic polymers in organic solvents. As is known to all, surface grafting is complicated and time-consuming with low active point density under rigorous conditions. Recently, Zhao41 et al. prepared zwitterionic surface via one-step sulfonation of PVDF and polyaniline (PANI) blend membrane. This obtained membrane surface exhibited excellent antifouling property. However, the total flux decline ratio (Rt) was still very high.
Poly(dimethylsiloxane) (PDMS), a kind of elastic polymer with low surface energy, can effectively release the adsorbed bio-contaminants since the freely rotating silicon–oxygen backbone impedes the possible formation of dipolar or hydrogen bonds with complementary functional groups of biofoulants.42 However, few studies focused on the combination of fouling releasing PDMS and fouling resistant zwitterionic materials are concerned about its application in membrane modification to improve antifouling performance before Zhao et al. reported the significant biofouling-resistance effect of amphiphilic copolymer containing poly(ethylene oxide) and PDMS segments recently.43
As a dissoluble polymer, poly(N,N-dimethylamino-2-ethylmethacrylate) (PDMAEMA)30 can be easily converted to quaternized PDMAEMA (PCBMA) which exhibits excellent antifouling property.44 Therefore, amphiphilic copolymers consisting of both PDMAEMA and PDMS segments seem to be promising in anti-fouling application, especially after quaternization.
In this work, a distinct simple method was introduced to fabricate novel antifouling PVDF membrane. A series of well-defined amphiphilic triblock copolymers PDMAEMA-b-PDMS-b-PDMAEMA were synthesized via atom transform radical polymerization.45,46 The enrichment of PDMAEMA segment on membrane surface was conductive to form zwitterionic poly(carboxybetaine methacrylate) by surface quaternization reaction (Scheme 1) while the hydrophobic segments PDMS acted as anchors in the matrix, which also effectively enhanced the anti-fouling property of PVDF membrane.
 | ||
| Scheme 1 Schematic illustration for the preparation and surface zwitterionicalization of the PVDF/PDMAEMA-b-PDMS-b-PDMAEMA blend membrane. | ||
2. Experiments
2.1. Materials and reagents
Hydroxypropyl polydimethylsiloxane (Mn = 4000 g mol−1) was purchased from Gelest Co. and used as received. Polyvinylidene fluoride (PVDF, MG-15) supplied by Arkema Company was dried at 100 °C for 24 hours before use. N,N-Dimethylamino-2-ethylmethacrylate (DMAEMA, 99%), dichloromethane (99.5%) and triethylamine (99.5%), copper(I) bromide (CuBr, 98%), cyclohexanone (99.5%), n-hexane (99.5%), N,N-dimethylformamide (DMF, 99.9%), tetrahydrofuran (THF, 99.8), calcium hydride (CaH2, 95%), neutral alumina (99.5%), magnesium sulfate (99.5%), N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA, 99%), 2-bromoisobutyryl bromide (BIBB, 98.0%), polyvinylpyrrolidone, bovine serum albumin (BSA, 98%) and 3-bromopropionic acid (3-BPA, 98.0%) were purchased from Sinopharm Chemical Reagent. N,N-Dimethylamino-2-ethylmethacrylate, tetrahydrofuran and triethylamine were dried over calcium hydride and distilled before use. Copper(I) bromide was stirred overnight in acetic acid, filtered, washed with ethanol and diethyl ether successively, dried in vacuum oven and restored under an argon atmosphere.2.2. Synthesis of macro-initiator (Br–PDMS–Br)
PDMS based macro-initiator was prepared according to our previous report.47 Hydroxy propyl polydimethylsiloxane (30 g, 7.5 mmol) and triethylamine (1.6 g, 15.5 mmol) were dissolved in 100 mL dry tetrahydrofuran in a three-neck round bottomed flask, which was immersed in an ice bath for 30 min before 2-bromoisobutyrate (3.5 g, 15 mmol) was dropwised to the stirred solution. The resulting mixture was stirred at room temperature for 16 h and filtered. The resulting liquid was dissolved in hexane (100 mL) and washed out with water. The bis(bromoalkyl)-terminated PDMS macro-initiator (Br–PDMS–Br) was obtained as a slightly yellow liquid with a yield of 90%. 1H NMR (400 MHz, CDCl3, Me4Si, δ): 0.00 (m, 6H), 0.079 (s, 6H, Si(CH3)2), 0.566 (m, 2H, CH2CH2CH2COO), 1.657 (m, 2H, CH2CH2COO), 1.74 (m, 6H, CBr(CH3)2), 4.36 (m, 2H, CH2COO).2.3. Synthesis of amphiphilic block copolymer poly(N,N-dimethylamino-2-ethylmethacrylate)-b-polydimethylsiloxane-b-poly(N,N-dimethylamino-2-ethylmethacrylate) by ATRP
Br–PDMS–Br macro-initiator (2 g, 0.5 mmol), N,N-dimethylamino-2-ethylmethacrylate (3.93 g, 25 mmol), toluene (12 g) and N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (0.1733 g, 1 mmol) were added to a Schlenk flask equipped with a magnetic stirring bar and degassed by one freeze–pump–thaw cycle. Before copper(I) bromide (0.1435 g, 1 mmol) was added into the flask under nitrogen atmosphere. After three freeze–pump–thaw circles, the flask was placed in an oil bath at 80 °C for 24 h. The reaction was stopped by exposing the solution to air, diluted with cyclohexanone, passed through a column of neutral alumina and precipitated in n-hexane. The dissolution and precipitation process were conducted three times. The obtained precipitate was dried in a vacuum oven at 60 °C for 24 h. The final product was white colloidal solid. The resulting triblock copolymer with different PDMAEMA unit amount, i.e. PDMAEMA17–PDMS–PDMAEMA17, PDMAEMA34–PDMS–PDMAEMA34, PDMAEMA64–PDMS–PDMAEMA64 was named as P17, P34 and P64. 1H NMR (400 MHz, CDCl3, Me4Si, δ): 0.00 (m, 6H), 0.088 (s, 6H, Si(CH3)2), 2.57 (m, 2H, CH2N(CH3)2), 4.05 (m, 2H, COOCH2), 2.29 (m, 6H, N(CH3)2).2.4. Fabrication of blend membrane
The flat blend membranes of PVDF and the block copolymer PDMAEMA-b-PDMS-b-PDMAEMA were prepared by the classical non-solvent induced phase inversion method. In the typical procedure, PVDF, PDMAEMA-b-PDMS-b-PDMAEMA and polyvinylpyrrolidone were dissolved in N,N-dimethylacetamide with a total concentration of 17 wt% plus 2 wt% of PVP under mechanical stirring at 60 °C. The mass ratio of the block copolymer to PVDF was kept at 0.1. The obtained solution was degassed for 12 h and then casted on a glass substrate with a blade to get a liquid layer with thickness about 200 μm, which was immersed into deionized water coagulation bath immediately and kept in the water bath until solvent free. Here the pure PVDF membrane and the blend membranes with the addition of P17, P34 and P64 were named as M0, M17, M34 and M64 respectively, and the blending membranes after the surface quaternization were named as M17-Q, M34-Q and M64-Q respectively. The details of membrane surface zwitterionicalization refers to ESI.†2.5. Copolymer characterization
2.6. Membrane characterization
2.7. Permeation and anti-fouling measurement
To investigate the antifouling property of the membranes, three-step filtration operations were conducted using BSA as a model pollutant. The pure water flux of the flat membranes with an effective area of 3.17 cm2 was recorded as JW1 at 0.1 MPa after being pre-pressed at 0.15 MPa for 0.5 hour. Then 1 g L−1 BSA solution was pumped to permeate the membrane following the same procedure as above, and flux was recorded as JP. After cleaning the fouling membrane with the deionized water, a second pure water flux (JW2) was tested. The permeation flux was calculated according to the following equation.
 | (1) |
The apparent rejection rate of the membrane was calculated according to the following equation:
 | (2) |
In order to test the long-term anti-fouling performance of modified PVDF membranes, multicycle operations were performed, where each membrane was pressurized at 0.15 MPa for 30 min and then the operation pressure was set at 0.1 MPa. Each cycle included three steps: first, deionized (DI) water was passed through the membrane for 30 min; second, the feed solution was replaced with 1.0 g L−1 BSA protein solution; third, the fouling membranes were washed with de-ionized water, and then immersed into the de-ionized water for 20 min. For each cycle, the deionized (DI) water flux JW was recorded, the flux of BSA solution JP was recorded. The dynamic filtration fouling test was performed as mentioned above.
To describe the anti-fouling property of the membranes, the flux recovery ratio (FRR), reversible flux decline ratio (Rr), irreversible flux decline ratio (Rir) and the total flux decline ratio (Rt) of protein pollution parameters were calculated according to the following equation:48
 | (3) |
 | (4) |
 | (5) |
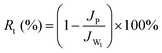 | (6) |
2.8. Protein adsorption
To qualitatively test the protein adsorption, protein adsorption study using FITC–BSA as a model foulant was prepared according to a reported procedure.49,50 In the protein adsorption test, a membrane sample (1 × 1 cm2) was immersed in 2 mL of PBS (pH 7.4) containing 0.1 g L−1 of FITC–BSA and shaken in a dark place for 12 h at 4 °C. Each sample was then rinsed with PBS three times to remove the free proteins. The adsorption of BSA–FITC on membrane sample was observed with a fluorescence microscope (Olympus BX-51, Japan).BCA (bicinchoninic acid) protein assay kit was used to quantitatively determine the adsorption of bovine serum albumin (BSA) and lysozyme (LZM) on membrane surface according to a similar method described by Yang et al.51 Briefly, pristine and modified membrane slides (1 × 1 cm2) were first immersed in phosphate buffer saline (PBS, pH 7.4) overnight at 4 °C and then immersed in 1 mL of PBS containing 1 mg mL−1 of BSA (or LZM) and shaken for 2 hours at 37 °C to reach the BSA adsorption equilibrium. After that, the membrane was rinsed three times with PBS gently to remove the free and loosely adsorbed proteins, and subsequently put into a tube containing 2 mL 2 wt% sodium dodecyl sulfate (SDS) in PBS and shaken for 2 hours at 37 °C to detach the adsorbed proteins. Eventually, 20 μL the washing solutions were transferred to 96-well plate. The protein concentration of the washing solutions was determined via the BCA protein Assay Reagent Kit. The experiment was conducted in triplicate. The amount of BSA (or LZM) was calculated by measuring the absorbance at 562 nm.
3. Results and discussion
3.1. Characterization of poly(N,N-dimethylamino-2-ethylmethacrylate)-b-polydimethylsiloxane-b-poly(N,N-dimethylamino-2-ethylmethacrylate) (PDMAEMA-b-PDMS-b-PDMAEMA) triblock copolymer
As shown in Fig. S1,† the distinctive signal at 0.09 ppm is assigned to –Si(CH3)2 group of PDMS, and the signal at 2.29 ppm is attributed to –N(CH3)2 group in PDMAEMA, which prove the successful reaction.The gel permeation chromatography (GPC) results of the PDMAEMA-b-PDMS-b-PDMAEMA copolymers in Table 1 and Fig. S2† show that the polydispersity indice (PDI) of PDMS macroinitiator is 1.14, the PDIs of all the copolymers are no more than 1.18, indicating that the polymerization is well controlled and a well-defined PDMAEMA-b-PDMS-b-PDMAEMA is obtained. The molecular weight of PDMAEMA-b-PDMS-b-PDMAEMA copolymers is increased from 8.2 to 20.1 kDa with increasing DMAEMA monomers.
| Sample ID | n (initiator)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n (monomer) n (monomer) |
Conversion (%) | Mn (kDa, GPC) | PDI |
|---|---|---|---|---|
| P17 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
51.2 | 8.2 | 1.17 |
| P34 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
65.2 | 12.2 | 1.14 |
| P64 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
62.3 | 20.1 | 1.18 |
3.2. Characterization of PDMAEMA-b-PDMS-b-PDMAEMA/PVDF blending membrane
As shown in Fig. S3,† a new absorption signal at 1726 cm−1 of M64 is attributed to the stretching vibration of the carbonyl groups of the PDMAEMA polymer segments, indicating the successful incorporation of the amphiphilic copolymer into the membrane. As compared with M64, the new absorption signal at 965 cm−1 for M64-Q belongs to the quaternary amine groups, as confirmed by Q. Zhang et al.52 The adsorption bands appeared at 1726 and 1592 cm−1 attribute to the stretching vibration of the carbonyl groups and carboxylate groups of the PCBMA polymer segments respectively, as confirmed by Q. Zhou et al.53 These results show that PDMAEMA segments in the blend membrane are successfully transformed to zwitterionic poly(carboxybetaine methacrylate) (PCBMA) after its reaction with 3-BPA. And the intensity of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch (1726 cm−1) for M64-Q is apparently higher than that for the M64, indicating the enrichment of PCBMA segments on the surface of M64-Q. It confirms that PDMAEMA segments in amphiphilic copolymers spontaneously migrate to the membrane surface, which is favorable for the improvement of wetting properties and fouling resistance.
O stretch (1726 cm−1) for M64-Q is apparently higher than that for the M64, indicating the enrichment of PCBMA segments on the surface of M64-Q. It confirms that PDMAEMA segments in amphiphilic copolymers spontaneously migrate to the membrane surface, which is favorable for the improvement of wetting properties and fouling resistance.
3.3. XPS characterization
The surface elements of the prepared M0, M64 and M64-Q are determined by XPS in Fig. 1 and the mass concentration is shown in Table 2. There are signals attributed to F, O and C elements in both M0 and M64, and two new signals in M64 and M64-Q are attributed to N and Si elements since N originates from amide groups in PDMAEMA segments and –N+(CH2)2(CH2)2COO− groups in PCBMA segments, and Si originates from PDMS segments, demonstrating the existence of the copolymer on the surface of the blend membrane. The N content in M64-Q is dramatically increased as compared with M64 and the N/F ratio is apparently higher than that in M64 (in Table 2), indicating the PCBMA segments on the surface of M64-Q are higher than on the surface of M64.| Membrane ID | Surface elemental mass concentration (%) | N/F ratio | ||||
|---|---|---|---|---|---|---|
| Carbon | Oxygen | Nitrogen | Fluorine | Silicon | ||
| M0 | 53.50 | 2.29 | 0 | 44.21 | 0 | 0 |
| M64 | 61.07 | 21.49 | 5.35 | 10.14 | 1.94 | 0.53 |
| M64-Q before rinsing | 60.35 | 23.68 | 6.96 | 3.18 | 5.84 | 2.19 |
| M64-Q after rinsing | 59.17 | 23.50 | 6.80 | 3.30 | 7.23 | 2.06 |
Meanwhile, the XPS N 1s core-level spectra of M64 and M64-Q in Fig. 2 shows that the peak at around 400.3 eV is ascribed to the nitrogen from tertiary amine in the PDMAEMA segments, and the peak appears at about 402.4 eV in the spectrum of M64-Q is the characteristic peak of quaternary amine nitrogen. This result obviously indicates that the tertiary amine in PDMAEMA segments is transformed into positively charged quaternary amine in PCBMA segments. Therefore, the above results prove that the PDMAEMA segments of amphiphilic copolymers migrate to the surface, and quaternization helps the further migration of zwitterionic PCBMA segments.
In order to prove the durability of the zwitterionic modified membrane, the chemical composition of the M64-Q before and after rinsing with water for 30 days is tested by XPS. As shown in Table 2, the composition of each element is similar before and after rinsing with water. In addition, the nitrogen peak at 400.3 eV from the amphiphilic triblock copolymer in Fig. 1 and the XPS N 1s core-level spectra of M64-Q remains similar after rinsing (Fig. 2). These results indicate the presence of the zwitterionic hydration layer on membrane surface even after 30 days of water rinsing, which can form renewable surface via the spontaneous migration to membrane surface to ensure excellent antifouling and durability despite of little elution.
3.4. Morphology of the membranes
The SEM images of the surfaces and cross-sections for M0, M17, M34 and M64 are shown in Fig. 3. The cross-section of all the membranes presents a typical asymmetric structure, where the finger-like macrovoids at the bottom of the blend membranes are developed more fully as compared to pure membrane since the addition of amphiphilic copolymers in the casting solution decrease the surface tension of the polymer phase and thus facilitate the mass inter-exchange between solvent and water in the process of phase inversion.54,55 The top surface morphology in Fig. 3(A) shows more pores with smaller pore size (Table 3) appears on the blend membranes while the pore size is gradually decreased with the increase of hydrophilic segments. This may be connected with the excellent compatibility and therefore the close-knit entanglement between PDMAEMA segments and the pore-forming agent polyvinylpyrrolidone, which hampers the diffusion of PVP into water during membrane formation process,56–58 resulting in the formation of smaller pore size on the blend membrane surface. Therefore, the longer PDMAEMA segments are, the smaller the pore size is. The top surface morphology in Fig. 3(C) shows that the pore size of the blend membranes after surface zwitterionicalization becomes smaller (Table 3) and rod-like micelles appear. There existed of large solubility difference between hydrophilic and hydrophobic segments in the amphiphilic copolymer, which can be easily self-assembled into micelles in a selective solvent.29,59 In the ethanol solution, micelles with core–shell structure are formed through the segregation of insoluble hydrophobic blocks into the core, which are surrounded by a shell composed of hydrophilic blocks PDMAEMA and thus drive the hydrophilic segments to migrate towards membrane surface. Thus, amphiphilic copolymers form rod-like micelles on the zwitterionic membrane surface, leading to blocking the membrane pores and decreasing the pore size. Furthermore, the movement activity of the segments is getting weaker, with the increasing hydrophilic segments, which leads to the shorter rod-like micelles of M64-Q than that of M17-Q and M34-Q.| Sample ID | M0 | M17 | M34 | M64 | M17-Q | M34-Q | M64-Q |
|---|---|---|---|---|---|---|---|
| Average pore size (nm) | 40.0 ± 3.2 | 36.2 ± 2.9 | 33.3 ± 2.8 | 29.2 ± 2.6 | 32.4 ± 2.4 | 30.5 ± 3.2 | 19.5 ± 4.4 |
| RMS (nm) | 22.3 | 20.8 | 25.2 | 30.8 | 31.6 | 32.3 | 40.4 |
Surface roughness has a great influence on membrane hydrophilicity as described by the well-known Wenzel equation. A rougher surface tends to have lower water contact angle for the hydrophilic surface, while an opposite phenomenon can be observed for the hydrophobic surface.60 Therefore, surface roughness of the membranes was characterized by AFM. As shown in Fig. 4, the surface of M17 becomes smoother than that of M0, which becomes rougher with further increasing PDMAEMA segments length since the roughness parameter RMS (Table 3) is increased from 22.3 nm for the M0 to 30.8 nm for M64. After zwitterionicalization, the hydrophilic and hydrophobic segments in the amphiphilic copolymer can be easily self-assembled into micelles. Therefore, the roughness parameter of the zwitterionic membranes is further increased to 40.4 nm for M64-Q.
 | ||
| Fig. 4 AFM characterization of the membranes M0, M17, M34, M64 and the blend membranes after quaternization. | ||
3.5. Membrane wettability measurement
As shown in Fig. 5(A), M0 has the maximum initial contact angle of 94°, which is noticeably decreased with the increase of the PDMAEMA segments for the blend membrane. It accounts for the fact that the enrichment of PDMAEMA segments on membrane surface and membrane pores improve the hydrophilicity of the blend membranes. The initial water contact angle of quaternized blend membranes is decreased from 81° to 75° with increasing PDMAEMA segments and the decreasing rate with time is much faster than that of the blend membranes. Particularly, the contact angle of M64-Q is dramatically decreased from 75° to 6° within 120 s while the water contact angle of the original PVDF membrane changes only about 5°, which is mainly because the zwitterionic PCBMA segments are able to form a hydration layer via electrostatic interaction and the hydrogen bonds to improve membrane surface hydrophilicity. It is accepted that, the stronger reaction between hydrophilic PDMAEMA segments and 3-BPA will not only change the surface chemical characteristics of the blend membranes, but also drive the hydrophilic segments to migrate towards membrane surface and membrane pore to improve hydrophilicity remarkably. Apart from the hydrophilic groups on the surface, the increased surface roughness of the modified membranes may also contribute to the improved wettability to some extent.3.6. Permeation and antifouling properties
Membrane flux can be affected by membrane pore structure, surface hydrophilicity, membrane materials, preparation conditions, etc.61 An increase in membrane hydrophilicity will always make the flux values larger due to the decreased interfacial strength between the membrane surface and water phase. Surface roughness may have both negative (more serious fouling) and positive (larger available membrane area) effects on membrane flux.62 However, it should be noted that water flux values of the membranes are mainly affected by the pore structures of the membranes (pore size, porosity, etc.). As shown in Fig. 6, the initial water flux (JW) of M0 is about 394.7 L m−2 h−1 while the initial water flux (JW) of blend membranes is decreased from 355.3 to 157.9 L m−2 h−1 with increasing PDMAEMA segments. The initial BSA flux (JBSA) of blend membranes is decreased from 86.8 to 73.2 L m−2 h−1 and BSA rejection is increased from 63% to 72%. This may be caused by the decrease of the effective pore size of blend membranes which arises from the enrichment PDMAEMA segments on membrane surface. However, both the initial BSA flux (JBSA) and BSA rejection of the blend membranes are higher than that of M0 since the hydrophilic segments on the membranes surface hinder the adsorption and deposition of BSA pollutant, and then decrease the water permeation resistance. After surface zwitterionicalization, all the quaternized blend membranes have lower JW, i.e. from 210.5 to 80.0 L m−2 h−1, the JBSA is decreased from 94.7 to 78 L m−2 h−1 and the BSA rejection is increased further with increasing PCBMA segments, and even gets up to 90% for the M64-Q, which can be ascribed to the transformation of PDMAEMA to PCBMA so as to drive the hydrophilic segments to migrate toward membrane surface, and then self-assemble into micelles. Therefore, the micelles and the thicker PCBMA layer on the surfaces block the pore size and further decrease the effective pore size, and the resulted zwitterionic PCBMA segments with BSA have the repulsive interaction to impede the adsorption and deposition of BSA pollutant.In order to comprehensively understand membrane fouling, two important parameters of water flux recovery ratio (FRR) and total flux decline ratio (Rt = Rr + Rir) are introduced and calculated based on the three fluxes, and the results are shown in Fig. 7. It's well known that lower relative flux reduction ratio (Rt) and higher flux recovery ratio (FRR) value mean better anti-fouling ability in membrane filtration. The total flux decline ratio (Rt) of pure membrane is 82%, while the Rt of blend membranes is decreased to 53.6% with increasing PDMAEMA segment length, which is further decreased to 14.5% after zwitterionization. Furthermore, the trend of the FRR values is in contrast to the Rt. The results indicate that the hydrophilicity and antifouling of blend membranes are improved dramatically via surface zwitterionicalization mediated by PDMAEMA segments of the amphiphilic polymer.
 | ||
| Fig. 7 Effect of the length of the PDMAEMA segments of amphiphilic polymer the total flux decline ratio (Rt) and the flux recovery ratio (FRR) of pure and blend PVDF membranes. | ||
In practical applications, periodic cleaning of membrane modules is a key step to wash out reversible BSA adsorption on membrane surface and to recover water flux. Therefore, durability test of the membranes was done by rinsing with water periodically. An antifouling membrane may recovery most of its initial water flux after rinsing with water. As shown in Fig. 8, the relative flux dates for all these membranes decrease slightly in the first 0.5 h of pure water permeation process. And the BSA flux is decreased sharply because of the formation of cake layer resulting from the deposition and adsorption of proteins on membrane surface and pores wall. It is worth noting that the blend membranes have lower flux decrease rate than M0 and the quaternized blend membranes have the lowest flux decrease rate, which are related with the membrane surface hydrophilicity. Especially, the decrease of the flux becomes lower with increasing hydrophilic segments. After 3 circle dynamic filtration fouling test, there is almost no decline in the water flux of zwitterionic membranes compared with that of M0, indicating a better antifouling property than that of M0. This is ascribed to the hydration of water molecules within the PCBMA segments and the hydrate layers can resist the adsorption and deposition of proteins on the membrane surface and membrane pore wall. It can be concluded that the anti-fouling ability of PVDF membrane is improved significantly by the surface zwitterionicalization of the amphiphilic copolymer addition. In order to prove the durability of the zwitterionic modified membrane, the time-dependent flux test is performed for 30 days. As shown in Fig. 8, both the water flux and BSA flux of M0 are decreased markedly with the increasing of time although the initial water flux (JW) of M0 is higher than all the modified membrane. However, the blend membranes and the quaternized blend membranes have lower flux decrease rate than M0, especially M64-Q. These results indicate excellent antifouling and durability due to the formation of renewable surface by the continuous migration of the internal amphiphilic copolymers as conformed by XPS.
 | ||
| Fig. 8 Time-dependent flux of the pure, blend membranes and the surface-zwitterionicalized blend membranes. | ||
As shown in Fig. 9(A), the pristine membrane surface demonstrates a maximum of the BSA–FITC protein adsorption, and the fluorescence intensities of the modified membranes are dramatically decreased with increasing PDMAEMA segments and further decreased after zwitterionization, especially for the M64-Q, since the hydrophilic PDMAEMA segments of the blend membranes and the hydration layer after zwitterionization endow the membranes with better antifouling capability than the pristine membrane. In addition, the adsorption amounts of bovine serum albumin (BSA) and lysozyme (LZM) on membrane surface in Fig. 9(B) show that the BSA adsorption is dramatically decreased from 111.62 to 4.12 μg cm−2 and the LZM adsorption is decreased from 135.29 to 17.75 μg cm−2 after zwitterionization, since the hydrophilicity of membrane surface is improved with increasing PDMAEMA segments and further improved after surface quaternization due to the transformation of PDMAEMA to PCBMA to form hydration layer. We all know that a more hydrophilic surface usually exhibits a lower amount of protein adsorption because of the electrostatic repulsive force. The results further confirm that the antifouling of zwitterionic membranes is improved which is in keeping with the hydrophilicity and antifouling performance discussed above.
The antifouling properties of the M64-Q/PVDF are further investigated by comparing with those of the state-of-the-art UF membranes in the literature. As shown in Table 4, most of the reported membranes based on the hydrophilic modification mechanism showed desirable FRR (74.5–94.0%) and rejection (81.0–92.5%) against BSA foulant and water permeability (14.0–595.4 L m−2 h−1 0.1 MPa−1). However, their Rt values were mostly higher than 20% and the amount of BSA adsorption was higher than 10 μg cm−2, indicating serious flux decline and BSA adhesion. Nonetheless, the resultant M64-Q/PVDF in this study exhibits outstanding antifouling performance against BSA foulants since its Rt value is as low as 14.5% and the BSA adsorption is 4.1 μg cm−2, which are superior to most of the UF membranes, indicating a very low flux decline and good resistance to protein adhesion. On the other hand, the FRR value, the BSA rejection and water permeability are similar to most of the UF membranes. These results indicate that the sustainable and eco-friendly antibiofouling zwitterionic membrane obtained via simple one-step method in this study exhibits greater fouling resistance than the reported UF membranes showed above, which is expected to be very competitive from both scientific and practical application viewpoints.
| Membrane name | BSA as a model foulant | ||||
|---|---|---|---|---|---|
| FRR (%) | Rt (%) | Rejection (%) | BSA adsorption (μg cm−2) | Water permeability (L m−2 h−1 0.1 MPa−1) | |
| a Values are obtained directly from the data in references.b Values are estimated from plots in references.c Values are calculated with the data or the plots in references. | |||||
| This work | 97.5 | 14.5 | 90.0 | 4.1 | 84.2 |
| Zwitterionic hydrogel thin films/PES35 | ∼84.0b | ∼50.0c | ∼90.0b | ∼23.0b | ∼40.0b |
| OMWCNTs–GO/PVDF25 | 85.1a | ∼75.0c | 86.9a | — | ∼140.0b |
| PDAAQ/rGO/PVDF63 | 86.8a | ∼62.5c | ∼87.5b | — | ∼56.0b |
| AgNPs–HNTs–rGO/PES64 | ∼80.0b | — | — | 37.5a | ∼69.0b |
| n-PANi65 | 91.0a | 11.0a | — | — | 86.9a |
| PDA–PSBMA/PLA39 | 94.0a | — | 90.0a | — | 14.0a |
| PVP-8%/PEI66 | 81.0a | — | — | 80.0a | 35.5a |
| Pluronic F127/PEI67 | 92.4a | ∼33.0b | 84.0a | — | 49.3a |
| rGO/TiO2/PVDF68 | 88.1a | ∼52.2c | — | — | ∼133.3b |
| MF-g-PEGn/PES29 | 91.6a | ∼35.0b | — | — | ∼100.0b |
| MHNTS/PEI69 | 74.5a | 86.2a | — | — | 44.6a |
| PEG/PES70 | — | — | — | ∼7.0b | — |
| Chitosan/BPPO71 | 85.1a | 58.3a | — | ∼17.0b | ∼300.0b |
| PANI–ES/PVDF41 | ∼85.0b | ∼40.0b | 90.0a | ∼15.0b | ∼120.0b |
| GO/TiO2/PVDF24 | 82.1a | ∼33.0b | 92.5a | — | 487.8a |
| OMWCNTs–GO/PVDF25 | 80.4a | ∼76.0b | ∼81.0b | — | 203.0a |
| Fe3O4/GO/PVDF26 | 86.4a | 31.5a | 92.0a | — | 595.4a |
4. Conclusions
The well-defined durable antifouling PVDF blend membrane was prepared by facile blending with amphiphilic triblock copolymer poly(N,N-dimethylamino-2-ethylmethacrylate)-b-polydimethylsiloxane-b-poly(N,N-dimethylamino-2-ethylmethacrylate) synthesized via atom transfer radical polymerization (ATRP), and then surface zwitterionicalization via one-step method. The flux recovery ratio and BSA rejection of the blend membranes are both increased and further increased after the quaternization. The hydrophilicity and antifouling properties of modified membranes are significantly improved especially after surface zwitterionicalization, since zwitterionic PDMAEMA segments on the membrane surface can form hydration layer via electrostatic interaction with water molecules, which also prevent the protein absorption. What's more, the elastic hydrophobic segments PDMS can act as anchors in the matrix, which effectively prevent the elution of amphiphilic polymer. The versatile one-step method represents a promising candidate for the future development of specialized high performance (antifouling, antibacterial and anticoagulant etc.) functional polymeric materials for applications in separation fields and biomedical fields.Acknowledgements
This work was supported by grants from National High-tech Research and Development Projects (863, 2012AA03A605), Program for New Century excellent talents (NCET-12-0827), the Natural Science Foundation of China (No. 51103019 and 21174027), the Program of Introducing Talents of Discipline to Universities (No. 111-2-04) and Chinese Universities Scientific Fund (DH-D-2013017).References
- F. Liu, N. A. Hashim, Y. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 375, 1–27 CrossRef CAS.
- X. Meng, W. Tang, L. Wang, X. Wang, D. Huang, H. Chen and N. Zhang, J. Membr. Sci., 2015, 487, 180–188 CrossRef CAS.
- S. T. Kelly and A. L. Zydney, J. Membr. Sci., 1995, 107, 115–127 CrossRef CAS.
- Z. Zhang, M. Zhang, S. Chen, T. A. Horbett, B. D. Ratner and S. Jiang, Biomaterials, 2008, 29, 4285–4291 CrossRef CAS PubMed.
- F. Qu, H. Liang, J. Zhou, J. Nan, S. Shao, J. Zhang and G. Li, J. Membr. Sci., 2014, 449, 58–66 CrossRef CAS.
- Q. Xu, Y. Ye, V. Chen and X. Wen, Water Res., 2015, 68, 182–193 CrossRef CAS PubMed.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef CAS PubMed.
- F.-Q. Nie, Z.-K. Xu, X.-J. Huang, P. Ye and J. Wu, Langmuir, 2003, 19, 9889–9895 CrossRef CAS.
- P.-F. Ren, Y. Fang, L.-S. Wan, X.-Y. Ye and Z.-K. Xu, J. Membr. Sci., 2015, 492, 249–256 CrossRef CAS.
- R. Zhang, Y. Su, X. Zhao, Y. Li, J. Zhao and Z. Jiang, J. Membr. Sci., 2014, 470, 9–17 CrossRef CAS.
- Q. Li, J. Imbrogno, G. Belfort and X.-L. Wang, J. Appl. Polym. Sci., 2015, 132 DOI:10.1002/app.41781.
- W.-P. Zhu, J. Gao, S.-P. Sun, S. Zhang and T.-S. Chung, J. Membr. Sci., 2015, 487, 117–126 CrossRef CAS.
- Q. Li, H.-H. Lin and X.-L. Wang, Journal, 2014, 4, 181–199 CrossRef.
- S. Jiang, J. Wang, J. Wu and Y. Chen, J. Appl. Polym. Sci., 2015, 132 DOI:10.1002/app.41726.
- J. Imbrogno, M. D. Williams and G. Belfort, ACS Appl. Mater. Interfaces, 2015, 7, 2385–2392 CAS.
- S. Boributh, A. Chanachai and R. Jiraratananon, J. Membr. Sci., 2009, 342, 97–104 CrossRef CAS.
- S. Yamamoto, S. Kitahata, A. Shimomura, K. Tokuda, T. Nishino and T. Maruyama, Langmuir, 2015, 31, 125–131 CrossRef CAS PubMed.
- Y. Chen, Q. Deng, J. Xiao, H. Nie, L. Wu, W. Zhou and B. Huang, Polymer, 2007, 48, 7604–7613 CrossRef CAS.
- Y.-C. Chiang, Y. Chang, A. Higuchi, W.-Y. Chen and R.-C. Ruaan, J. Membr. Sci., 2009, 339, 151–159 CrossRef CAS.
- S. Van Geluwe, C. Vinckier, L. Braeken and B. Van der Bruggen, J. Membr. Sci., 2011, 378, 128–137 CrossRef CAS.
- Y.-F. Yang, Y. Li, Q.-L. Li, L.-S. Wan and Z.-K. Xu, J. Membr. Sci., 2010, 362, 255–264 CrossRef CAS.
- Z. Yi, C.-J. Liu, L.-P. Zhu and Y.-Y. Xu, Langmuir, 2015, 31, 7970–7979 CrossRef CAS PubMed.
- J. Yin and J. Zhou, Desalination, 2015, 365, 46–56 CrossRef CAS.
- Z. Xu, T. Wu, J. Shi, K. Teng, W. Wang, M. Ma, J. Li, X. Qian, C. Li and J. Fan, J. Membr. Sci., 2016, 520, 281–293 CrossRef CAS.
- J. Zhang, Z. Xu, W. Mai, C. Min, B. Zhou, M. Shan, Y. Li, C. Yang, Z. Wang and X. Qian, J. Mater. Chem. A, 2013, 1, 3101–3111 CAS.
- Z. Xu, T. Wu, J. Shi, W. Wang, K. Teng, X. Qian, M. Shan, H. Deng, X. Tian, C. Li and F. Li, ACS Appl. Mater. Interfaces, 2016, 8, 18418–18429 CAS.
- G.-d. Kang and Y.-m. Cao, J. Membr. Sci., 2014, 463, 145–165 CrossRef CAS.
- D. Liu, D. Li, D. Du, X. Zhao, A. Qin, X. Li and C. He, J. Membr. Sci., 2015, 493, 243–251 CrossRef CAS.
- Y. Liu, Y. Su, X. Zhao, Y. Li, R. Zhang and Z. Jiang, J. Membr. Sci., 2015, 486, 195–206 CrossRef CAS.
- Z. Yi, L.-P. Zhu, Y.-F. Zhao, Z.-B. Wang, B.-K. Zhu and Y.-Y. Xu, J. Membr. Sci., 2014, 463, 49–57 CrossRef CAS.
- Y. Xie, M. Liu and J. Zhou, Appl. Surf. Sci., 2012, 258, 8153–8159 CrossRef CAS.
- Q. Shao, Y. He, A. D. White and S. Jiang, J. Phys. Chem. B, 2010, 114, 16625–16631 CrossRef CAS PubMed.
- T. Kondo, K. Nomura, M. Murou, M. Gemmei-Ide, H. Kitano, H. Noguchi, K. Uosaki, K. Ohno and Y. Saruwatari, Colloids Surf., B, 2012, 100, 126–132 CrossRef CAS PubMed.
- W. Ogieglo, J. de Grooth, H. Wormeester, M. Wessling, K. Nijmeijer and N. E. Benes, Thin Solid Films, 2013, 545, 320–326 CrossRef CAS.
- Y.-F. Zhao, L.-P. Zhu, Z. Yi, B.-K. Zhu and Y.-Y. Xu, J. Membr. Sci., 2014, 470, 148–158 CrossRef CAS.
- Y.-F. Zhao, P.-B. Zhang, J. Sun, C.-J. Liu, Z. Yi, L.-P. Zhu and Y.-Y. Xu, J. Colloid Interface Sci., 2015, 448, 380–388 CrossRef CAS PubMed.
- Y.-F. Zhao, L.-P. Zhu, Z. Yi, B.-K. Zhu and Y.-Y. Xu, J. Membr. Sci., 2013, 440, 40–47 CrossRef CAS.
- J.-J. Wang, M.-B. Wu, T. Xiang, R. Wang, S.-D. Sun and C.-S. Zhao, J. Appl. Polym. Sci., 2015, 132 DOI:10.1002/app.41585.
- L.-J. Zhu, F. Liu, X.-M. Yu, A.-L. Gao and L.-X. Xue, J. Membr. Sci., 2015, 475, 469–479 CrossRef CAS.
- X. He, W. Zhou, X. Xu and W. Yang, Prog. Chem., 2013, 25, 1023–1030 CAS.
- X. Zhao and C. He, ACS Appl. Mater. Interfaces, 2015, 7, 17947–17953 CAS.
- J. E. Mark, Acc. Chem. Res., 2004, 37, 946–953 CrossRef CAS PubMed.
- X. Zhao, Y. Su, Y. Li, R. Zhang, J. Zhao and Z. Jiang, J. Membr. Sci., 2014, 450, 111–123 CrossRef CAS.
- C. Ma, H. Zhou, B. Wu and G.-Z. Zhang, ACS Appl. Mater. Interfaces, 2011, 3, 455–461 CAS.
- X. Sun, H. Zhang, X. Huang, X. Wang and Q.-F. Zhou, Polymer, 2005, 46, 5251–5257 CrossRef CAS.
- H. Huang, L. He, K. Huang and M. Gao, J. Colloid Interface Sci., 2014, 433, 133–140 CrossRef CAS PubMed.
- J. Xu, M. Qiu, B. Ma and C. He, ACS Appl. Mater. Interfaces, 2014, 6, 15283–15290 CAS.
- J. Wang, W.-Z. Lang, H.-P. Xu, X. Zhang and Y.-J. Guo, Chem. Eng. J., 2015, 260, 90–98 CrossRef CAS.
- A. S. Blawas, T. F. Oliver, M. C. Pirrung and W. M. Reichert, Langmuir, 1998, 14, 4243–4250 CrossRef CAS.
- J. B. Delehanty, K. M. Shaffer and B. Lin, Anal. Chem., 2004, 76, 7323–7328 CrossRef CAS PubMed.
- C. Yang, X. Ding, R. J. Ono, H. Lee, L. Y. Hsu, Y. W. Tong, J. Hedrick and Y. Y. Yang, Adv. Mater., 2014, 26, 7346–7351 CrossRef CAS PubMed.
- Q. Zhang, N. Gao, Y. Yang, Y. K. Gong, Y. B. Wang, S. P. Zhang and S. Q. Shi, Chem. J. Chin. Univ., 2013, 34, 1270–1276 CAS.
- Q. Zhou, X.-P. Lei, J.-H. Li, B.-F. Yan and Q.-Q. Zhang, Desalination, 2014, 337, 6–15 CrossRef CAS.
- Y.-H. Zhao, Y.-L. Qian, B.-K. Zhu and Y.-Y. Xu, J. Membr. Sci., 2008, 310, 567–576 CrossRef CAS.
- S. Liu, Z. Jiang and X. He, J. Membr. Sci., 2013, 442, 97–106 CrossRef CAS.
- J. F. Hester, P. Banerjee and A. M. Mayes, Macromolecules, 1999, 32, 1643–1650 CrossRef CAS.
- D. Wang, K. Li and W. K. Teo, J. Membr. Sci., 1999, 163, 211–220 CrossRef CAS.
- M. L. Yeow, Y. T. Liu and K. Li, J. Appl. Polym. Sci., 2004, 92, 1782–1789 CrossRef CAS.
- H. Otsuka, Y. Nagasaki and K. Kataoka, Adv. Drug Delivery Rev., 2012, 64, 246–255 CrossRef.
- Z. Wang, M. Elimelech and S. Lin, Environ. Sci. Technol., 2016, 50, 2132–2150 CrossRef CAS PubMed.
- S. H. Woo, J. Park and B. R. Min, Sep. Purif. Technol., 2015, 146, 187–191 CrossRef CAS.
- R. Kumar and A. F. Ismail, J. Appl. Polym. Sci., 2015, 132(21) DOI:10.1002/app.42042.
- H. Liu, G. Zhang, C. Zhao, J. Liu and F. Yang, J. Mater. Chem. A, 2015, 3, 20277–20287 CAS.
- Q. Zhao, J. Hou, J. Shen, J. Liu and Y. Zhang, J. Mater. Chem. A, 2015, 3, 18696–18705 CAS.
- X. Huang, B. T. McVerry, C. Marambio-Jones, M. C. Y. Wong, E. M. V. Hoek and R. B. Kaner, J. Mater. Chem. A, 2015, 3, 8725–8733 CAS.
- P. Kanagaraj, N. Alagumalai, D. Rana, T. Matsuura, S. Neelakandan and K. Malarvizhi, Ind. Eng. Chem. Res., 2015, 54, 4832–4838 CrossRef CAS.
- P. Kanagaraj, S. Neelakandan, A. Nagendran, D. Rana, T. Matsurra and M. Shalini, Ind. Eng. Chem. Res., 2015, 11628–11634 CrossRef CAS.
- M. Safarpour, A. Khataee and V. Vatanpour, Ind. Eng. Chem. Res., 2014, 53, 13370–13382 CrossRef CAS.
- R. S. Hebbar, A. M. Isloor, K. Ananda and A. F. Ismail, J. Mater. Chem. A, 2016, 4, 764–774 CAS.
- J. Deng, X. Liu, S. Zhang, C. Cheng, C. Nie and C. Zhao, Langmuir, 2015, 31, 9665–9674 CrossRef CAS PubMed.
- Y. Feng, X. Lin, H. Li, L. He, T. Sridhar, A. K. Suresh, J. Bellare and H. Wang, Ind. Eng. Chem. Res., 2014, 53, 14974–14981 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20079f |
| This journal is © The Royal Society of Chemistry 2016 |






