Effects of magnetization on Fe(III) species in magnetically enhanced coagulation ultrafiltration processes
Jie Wang*ab,
Sasa Yangb,
Hui Jiaa and
Hongwei Zhangab
aState Key Laboratory of Separation Membranes and Membrane Processes, Tianjin Polytechnic University, Tianjin 300387, China. E-mail: wangjie@tjpu.edu.cn; Fax: +86 022 8395 5668; Tel: +86 022 8395 5668
bSchool of Environmental and Chemical Engineering, Tianjin Polytechnic University, Tianjin 300387, China
First published on 1st September 2016
Abstract
The effects of magnetization on floc properties and membrane fouling in magnetically enhanced coagulation ultrafiltration (MEC-UF) processes for micro-polluted water treatment were investigated in this study. The results showed that the magnetized seeds could influence the distributions of Fe(III) species due to their residual magnetic flux density after magnetization. The hydrolysis products of Fe(III) were affected by the Fe3O4 suspension at various magnetization times, which influenced the coagulation performance and floc properties of Fe(III). The magnetized Fe3O4 suspension not only increased the quantity of particles in the micro-polluted water but also affected the sum of Fea and Feb of Fe(III), especially Fea. However, online measurement of Fe(III) species showed that the MEC-UF process with 4 min magnetization made the best use of Fea. Therefore, the flocs of MEC-UF were much larger with higher strength factor and recovery factor, and the cake layers were looser and more porous. This leads to a better knowledge about magnetically enhanced coagulation for membrane fouling reduction.
1 Introduction
Ultrafiltration (UF) membrane processes have been widely used in micro-polluted water treatment.1,2 However, membrane fouling is the main impediment for wide application of these processes. Hence, coagulation, for removing the colloidal matter as an indispensable pretreatment process, has been extensively adopted in membrane filtration processes due to its low operation costs and effective membrane fouling reduction.2According to previous studies, many approaches have been developed for enhancing coagulation, such as increase in coagulant dosage, acidification of raw water and application of new composite coagulants.3,4 Mao et al.5 investigated the application of polyferric chloride (PFC) and polyferric chloride–polydimethyldiallylammonium chloride (PFC–PDMDAAC) to enhance coagulation by increasing dosage and applying new composite coagulants, results showed that both approaches can improve the coagulation efficiency. However, membrane fouling index (MFI) of PFC–PDMDAAC was significantly higher than that of PFC, indicating that the increased dosage could effectively mitigate membrane fouling, while membrane fouling was exaggerated by adding the new composite coagulant. Therefore, it is necessary to find more effective coagulation technologies to simultaneously improve coagulation performance and reduce membrane fouling.
Coagulant combined with magnetic seeds induces the so-called magnetically enhanced coagulation (MEC) owing to its ability of formation of larger flocs with higher fractal dimension and better coagulation performance than conventional coagulation.6 Many previous studies have revealed that the physical and chemical properties of both pollutants and coagulants were influenced by magnetic particles.7,8 Liu et al.9 studied the removal effects on algal cells and dissolved organics in water via a magnetic coagulant synthesized by compounding acid-modified fly ash with magnetite (Fe3O4), the mechanism of algal removal explored showed that the magnetic coagulant played multiple roles in mesoporous adsorption, netting, bridging and etc. Mohammed et al.10 introduced a novel method by integrating magnetic field exposure with adsorption technique. The results showed that adsorption performance and rate could be enhanced by magnetic field and the magnetization process accelerated the removals of colour, TSS and COD in adsorption process. Besides, membrane fouling was more significantly alleviated via MEC with the presence of ferromagnetic seeds which magnetized for 5 min. It was ascribed to the fact that porous cake layer with flocs with large size was able to retard decline rate of membrane flux.11 However, the effect of magnetized seeds on coagulant was unknown and the reason why MEC could form loose and porous cake layer is not clear. Additionally, the studies related to positive effects of the MEC on membrane fouling reduction are still lacking.
It has been demonstrated that membrane fouling was affected by floc characteristics based on the physical and chemical properties of coagulant hydrolysis products.12 The distributions of Fe(III) species could be measured by a timed complexation spectroscopy method. The Fe(III) species varied over different reaction times between Fe(III) and Ferron (8-hydroxy-7-iodoquinoline-5-sulfonic acid), such as reaction within 1 min (Fea: Fe3+, FeOH2+, Fe(OH)2+), reaction over 3 h (Feb: [Fe2(OH)2]4+, [Fe3(OH)4]5+, [Fe5(OH)9]6+) and no reaction (Fec: [Fe12(OH)34]2+). Fe(III) that reacted with Ferron could form green metal complexes, and they could be measured at a wavelength of 600 nm by an ultraviolet-visible spectrophotometer to quantify the amounts of Fe(III) species (the absorbance of the green metal complex and Fe(III) concentration has notable positive correlation).13,14 The total amount of Fe(III) [Fe(T)] was measured after acidification by hydrochloric acid.
Dong et al.15 investigated the relationship between Fe(III) species and micro-filtration membrane performance to explore the effects of basicity (the moles of base added and/or bounded to the moles of Fe3+([OH−]/[Fe3+])) on Fe(III) species. The results indicated that more Fea and Feb in PFC favored the formation of larger and looser flocs, resulting in less membrane fouling. Another report of Dong et al.16 showed similar findings that the degree of pH dependent was strongly correlated with the distributions of Fe(III) species. What's more, Fea and Feb in coagulants were good for membrane fouling reduction. Hence, various factors impacted the Fe(III) species.
In a word, it was obvious that magnetization might influence the Fe(III) species. This study aimed to determine the effects of Fe3O4 suspension after magnetization on the distributions of Fe(III) species, and investigated the influences of magnetized seeds on coagulation performance, floc properties and membrane fouling of magnetically enhanced coagulation ultrafiltration (MEC-UF) process. Moreover, the objective of this study was to analyze the mechanism of membrane fouling reduction in MEC-UF process.
2 Materials and methods
2.1 Raw water
The micro-polluted water employed in this study was taken from Luanhe River. The pH, turbidity, UV254, zeta potential and conductivity were measured using a pH meter (PHS-3C, China), a turbidimeter (Hach 2100P, USA), an ultraviolet-visible spectrophotometer (Cary60, Agilent, USA), a zetasizer (ZS90, Malvern, UK) and a conductivity meter (DDSJ-308A, China), respectively. And the sample was filtered through a 0.45 μm filter membrane, before determination of TOC using a combustion-type organic carbon analyzer (TOC-Vcph analyzer, Shimadzu, Japan). The characteristics of raw water are shown in Table 1.| Parameter | Unit | Value |
|---|---|---|
| pH | — | 8.46–8.55 |
| Turbidity | NTU | 10.1–15.4 |
| UV254 | cm−1 | 0.1653–0.1909 |
| TOC | mg L−1 | 10.57–11.16 |
| Zeta potential | mV | −14.7–−15.5 |
| Conductivity | μs cm−1 | 50.1–58.6 |
| Cl− | mg L−1 | 150.2–152.4 |
| NO3− | mg L−1 | 20.15–22.54 |
2.2 Coagulant
Although colloidal particles are steady in raw water with negative surface charges, they can be destabilized by adding coagulant.17 In this study, FeCl3 combined with Fe3O4 was chosen as the coagulant and the magnetic seeds as they could promote the formation of loose and porous cake layer, which significantly reduced membrane fouling. The FeCl3 solution was prepared by dissolving FeCl3·6H2O (Kermel, Tianjin, China) into the deionized water, while obtaining the Fe3O4 suspension by employing Fe3O4 (Kermel, Tianjin, China) with diameter of 40–60 μm. In this study, the Fe3O4 suspension was magnetized in magnetic field intensity (Beijing Ch-Hall electronic devices Co., Ltd, China) H = 0.2 T with different times.2.3 Jar tests
The jar tests were performed in a 1.0 L beaker using a programmable lab-scale jar test apparatus (ZR4-6, Zhongrun Water Industry Technology Development Co. Ltd, China) to investigate the performance of the MEC process. The FeCl3 solution and the magnetized Fe3O4 suspension were added in the dosing tube and dosed after the raw water was stirred at 200 rpm for 30 s, and then the solutions were stirred with a rapid of 200 rpm for 1 min, followed by a slow stirring with a rapid of 60 rpm for 15 min. After 20 min settling, the water samples were collected at the depth of 2.0 cm below the surface to determine the qualities.2.4 Floc properties
Coagulation experiments were carried out in the same manner as the jar tests mentioned above to examine the structure and strength of flocs under different conditions. The solutions were stirred at 200 rpm for 1 min, followed by a slow stir at 60 rpm for 13 min. Then another rapid stir of 200 rpm was conducted for 3 min to break the flocs, after which regrowth phase of flocs was obtained with a slow stir for 13 min. During this process, a laser diffraction instrument (Mastersizer 2000, Malvern, UK) was used to monitor the dynamic flocs size including formation, breakage and regrowth of flocs. The mean size (d50) measurement was implemented every 1 min during the jar tests. Floc strength and recovery ability were quantitatively evaluated by strength factor (Sf) and recovery factor (Rf) and calculated as follows:
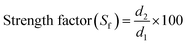 | (1) |
 | (2) |
2.5 Filtration setup
All filtration tests were performed using polyvinylidene fluoride (PVDF) hollow fiber membranes (Tianjin MOTIMO Membrane Technology CO., Ltd, China) with a mean pore size of about 0.1 μm. The membrane permeability measured with deionized water (TOC < 0.1 mg L−1, pH 6.5 and conductivity of around 1 μs cm−1) was 20 L (m2 h)−1 at the pressure of 0.02 MPa. A new membrane was used for each filtration test.The membrane filtration setup in this study was designed at a constant flux of 20 L (m2 h)−1. MEC-UF experiments were carried out in a dead-end mode. The operating temperature was 20 ± 1 °C. Raw water mixed with FeCl3 and Fe3O4 was continuously pumped into the membrane reactor. The membrane filtration system was operated for 5 h, after which the fouled membrane was physically washed with high pressure water for 2 min.19 This process was carried out for two cycles under the same operating conditions. The transmembrane pressure (TMP) change during the whole filtration was recorded using a pressure transmitter (Danfoss, MBS 3000, Denmark) connected to a paperless recording instrument (LCKLY, XSW10R, China) (Fig. 1).
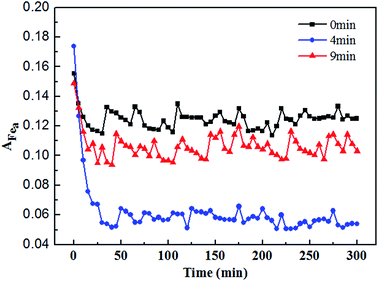
During the first membrane filtration process, the Fea of Fe(III), which was affected by magnetized Fe3O4 suspension at different magnetization times, was measured online for 5 h using an ultraviolet-visible spectrophotometer (UV-6300, Shanghai MAPADA Instruments Co., Ltd, China) in parallel. The coagulation solution was filtered by a 0.45 μm filter membrane before reacting with the Ferron solution, and a wider pipeline was used to make sure that there was no bubble in the whole measurement process. Therefore, the results were accurate and stable. The Fea content could be measured after 1 min reaction between the filtered water and the Ferron solution with the ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In this study, the Ferron solution was prepared by mixing 0.2% Ferron (Chengxin, Shanghai, China), 20% NaAc (Kermel, Tianjin, China), and 1
1. In this study, the Ferron solution was prepared by mixing 0.2% Ferron (Chengxin, Shanghai, China), 20% NaAc (Kermel, Tianjin, China), and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 HCl (Kermel, Tianjin, China) at the ratio of 5
9 HCl (Kermel, Tianjin, China) at the ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.14
2.14
3 Results and discussion
3.1 Effects of magnetization times on Fe(III) species
The Fe3O4 suspension was magnetized from 1 to 10 min to determine the effects of magnetization times on Fe(III) species, and fresh Fe3O4 suspension was used as reference suspension. 5 mL of Fe3O4 suspensions at a concentration of 10 mg mL−1 was conducted at different magnetization times with the magnetic field intensity H = 0.2 T. Subsequently, the above-mentioned Fe3O4 suspension was mixed with the same volume of 10 mg mL−1 FeCl3. After that, Fe(III) influenced by magnetized Fe3O4 reacted with Ferron. As shown in Table 2 and Fig. 2, there was notable connection between Fe(III) species and the residual magnetic flux density of magnetized Fe3O4 suspensions with different magnetization times. It implied that the residual magnetic flux densities of Fe3O4 suspensions with different magnetization times might exert some effects on the distributions of Fe(III) species. When the magnetization time was 4 min, the quantities of Fea and Feb reached the peak values, accounting for up to 92.9%, and then decreased as the magnetization time increased, with Fea and Feb of 74% for 9 min of magnetization. As displayed in Fig. 2, the residual magnetic flux densities (Model 1600 Gauss/Tesla meter, Beijing, China) of magnetized Fe3O4 suspensions changed over time, which might be due to interaction among those magnetized seeds. Finally, it maintained at a stable level. However, the residual magnetic flux density of 0.92 mT after 4 min magnetization was larger than that after 9 min (about 0.21 mT) during the whole procedure. Hence, due to the distributions of Fe(III) species, the magnetization times of Fe3O4 suspension used in the following experiments were 4 and 9 min.| Magnetization time/min | Fea (%) | Feb (%) | Fec (%) |
|---|---|---|---|
| 0 | 55.6 | 16.9 | 27.5 |
| 1 | 58.4 | 17.0 | 24.6 |
| 2 | 62.8 | 16.0 | 21.2 |
| 3 | 65.4 | 18.4 | 16.2 |
| 4 | 73.7 | 19.2 | 7.1 |
| 5 | 66.1 | 18.5 | 15.4 |
| 6 | 63.0 | 17.6 | 19.4 |
| 7 | 61.4 | 15.8 | 22.8 |
| 8 | 59.2 | 16.2 | 24.6 |
| 9 | 57.9 | 16.1 | 26.0 |
| 10 | 58.0 | 16.1 | 25.9 |
3.2 The hydrolysis products of Fe(III) affected by magnetized seeds
A series of Fe(III) hydrolysis reactions took place in the water, so the pollutants could be removed through the interaction between the pollutants and Fe(III) hydrolysis products. In Fig. 3, hydrolysis products of Fe(III) affected by Fe3O4 suspension with 4 and 9 min magnetization were investigated by an ultraviolet-visible spectrophotometer with the wavelength ranging from 200 to 700 nm. The absorption peaks were obtained at the wavelengths of 297 and 205 nm, both of which were the characteristic wavelengths of Fe(OH)2+ that belongs to Fea. The quantity of Fea was in the order of 4 min > 9 min > 0 min, which demonstrated the effects of the Fe3O4 suspension magnetization on Fe(III) species. | ||
| Fig. 3 UV-vis absorbance spectra of the hydrolysis products of Fe(III) at different magnetization times. | ||
3.3 Coagulation performance
UV254, TOC removal efficiency and zeta potential of FeCl3 at different magnetization times as a function of Fe3O4 dosage were comparatively evaluated, and the results are shown in Fig. 4. Zeta potential, TOC concentration and UV254 of raw micro-polluted water were less than −15.5 mV, and close to 11.16 mg L−1 and 0.1909 cm−1, respectively. At different magnetization times, FeCl3 dosages were 10 and 20 mg L−1, whilst applying 2, 4, 6, 8 and 10 mg L−1 of the Fe3O4 suspension. As shown in Fig. 4, at FeCl3 dosage of 10 mg L−1, zeta potential increased with an increase in the Fe3O4 dose. On the contrary, the UV254 and TOC removals decreased and reached a plateau afterwards when the dosage of Fe3O4 suspension was 4 mg L−1. The same results could be found with FeCl3 dosage of 20 mg L−1 and Fe3O4 suspension dosage of 6 mg L−1. The coagulation performance for 20 mg L−1 of FeCl3 was better than that for 10 mg L−1. | ||
| Fig. 4 Coagulation performance of FeCl3 as a function of the Fe3O4 suspension dosages and magnetization times of Fe3O4 suspension. | ||
In addition, the coagulation performance showed slight difference when changing magnetization times. The best coagulation performance was attained at the magnetization time of 4 min, the result was in agreement with Gu et al.,20 who reported that COD and color removal efficiencies decreased along with the extension of magnetization time in papermaking wastewater treatment via coagulation with magnetized polymetric ferric sulfate. More specifically, zeta potential of −7.06 mV was higher than that of −9.51 and −9.10 mV at the same dosage when magnetizing for 0 and 9 min, respectively. Thus, the charge neutralization ability of Fe3O4 suspension was the highest with 4 min of magnetization. The results revealed that the charge neutralization ability of FeCl3 was affected by the magnetization time because of different distributions of Fe(III) species. Comparing to the magnetization time of 9 min, the amounts of Fea and Feb were larger and the corresponding value of Fec was lower at the magnetization time of 4 min. Fea and Feb were highly positively charged monomers and polymeric species, which caused efficient charge neutralization with the negatively charged colloids.21
Base on the coagulation performances, the experiments regarding floc properties and membrane fouling would be conducted employing 10 mg L−1 of FeCl3 combined with 4 mg L−1 of Fe3O4 suspension and 20 mg L−1 of FeCl3 combined with 6 mg L−1 of Fe3O4 suspension at magnetization times of 0, 4, and 9 min.
3.4 Floc properties
At lower (10 mg L−1) and higher FeCl3 dosage (20 mg L−1) combined with the Fe3O4 suspension dosages of 4 and 6 mg L−1, respectively, the curves of floc growth, breakage and regrowth with the Fe3O4 suspension magnetization times of 0, 4 and 9 min are shown in Fig. 5, where the floc size was represented by median equivalent diameter (d50). The flocs grew quickly once the coagulant was dosed into the raw water due to the rapid mixing. When the floc growth and breakage achieved a balance, the floc size grew slowly and could reach a plateau. The floc size obtained at the FeCl3 dosage of 20 mg L−1 combined with 6 mg L−1 Fe3O4 suspension was around 600 μm, which was markedly larger than that at 10 mg L−1 FeCl3 combined with 4 mg L−1 Fe3O4. Additionally, the floc sizes showed difference at various magnetization times even though with the same coagulant dosage, and they also followed the sequence of 4 min > 9 min > 0 min arising from impacts of the contents of Fe(III) species on floc properties. | ||
| Fig. 5 Influences of magnetization times on floc formation, breakage and regrowth in terms of floc size (d50) at different FeCl3–Fe3O4 dosages. | ||
It was observed that the floc size was in the order: bridging > sweep > charge neutralization.17 FeCl3 contained more Fec serving as larger polymer or colloidal species, which could absorb or bridge organic matters to form larger flocs. However, when Fe3O4 suspension was magnetized for 4 min, FeCl3 removed more UV254 and TOC with 73.7% of Fea and 19.2% of Feb than those at others magnetization times. In addition, larger amounts of colloids were neutralized by FeCl3, resulting in larger floc size. The results were in accordance with Dong et al.,21 who revealed that Fe(III) salts with more Fea and Feb produced larger aggregates.
To quantitatively evaluate the floc properties, the mean floc size (d50) was compared during the growth, breakage and regrowth period for different conditions (Fig. 6). The Sf and Rf were calculated according to eqn (1) and (2), and the results are shown in Table 3. The Sf and Rf still conformed to the order: 4 min > 9 min > 0 min, regardless of the coagulant dosages. As reported by Dong et al.21 that FeCl3 flocs possessing larger amounts of Fea and Feb were more sensitive to various shear rates than the others coagulants with more Fec. It suggested that the coagulants with more Fea and Feb had poorer strength factor, which seemed to be contrary to the findings of this study. However, when the FeCl3 was combined with Fe3O4 suspension as coagulant, small Fe3O4 particles functioned as the core target. Subsequently, more flocs were adsorbed on the particles, resulting in the formation of larger flocs with higher floc strength.
 | ||
| Fig. 6 Particle size distributions of flocs generated at different FeCl3–Fe3O4 dosages and magnetization times during formation, breakage and regrowth period. | ||
| Number | Sf | Rf |
|---|---|---|
| A0 | 50.22 | 71.10 |
| A4 | 53.76 | 85.13 |
| A9 | 50.95 | 80.62 |
| B0 | 58.02 | 80.25 |
| B4 | 61.57 | 86.73 |
| B9 | 58.06 | 82.68 |
3.5 Magnetically enhanced coagulation ultrafiltration process
4 Conclusions
The specific conclusion in this study could be drawn as follows:(1) The distributions of Fe(III) species had a strong correlation with the residual magnetic flux density of the magnetized Fe3O4 particles. The species distributions and hydrolysis products of Fe(III) could be affected by the Fe3O4 suspension due to its residual magnetic flux density after magnetization.
(2) Owing to the higher amounts of Fea and Feb, FeCl3 combined with Fe3O4 suspension of 4 min magnetization achieve better coagulation performance, and the floc size was larger with higher strength factor and recovery factor.
(3) The results from online measurement of Fe(III) species revealed that more effective utilization of Fea during MEC-UF process with 4 min of magnetization realized better membrane fouling reduction.
Effects of the Fe3O4 suspension magnetization on the distributions of Fe(III) species could provide a new perspective in analyzing the mechanism of membrane fouling reduction in MEC-UF process.
Acknowledgements
This study is financially supported by the National Natural Science Foundation of China (No. 51578375, No. 51378349), Science and Technology Planning Project of Tianjin, China (15PTSYJC00230, 14ZCDGSF00128), and Program for Changjiang Scholars and Innovative Research Team in University (Grand No. IRT13084).References
- J. M. Laine, D. Vial and P. Moula, Desalination, 2000, 131, 17–25 CrossRef CAS.
- J. G. Jacangelo, R. R. Tussel and M. Waston, Desalination, 1997, 113, 119 CrossRef CAS.
- G. P. Crozes, P. White and M. Marshall, J.–Am. Water Works Assoc., 1995, 87, 78–89 CAS.
- M. Q. Yan, D. S. Wang, J. H. Qu, J. R. Ni and C. W. K. Chow, Water Res., 2008, 42, 2278–2286 CrossRef CAS PubMed.
- R. R. Mao, Y. Wang, B. Zhang, W. Y. Xu, M. Dong and B. Y. Gao, Desalination, 2013, 314, 161–168 CrossRef CAS.
- J. Wang, L. L. Liu, J. Yang, S. S. Yang, H. W. Zhang, H. Jia and X. F. Guo, Water Sci. Technol.: Water Supply, 2016, 16, 104–114 CrossRef.
- H. Ozak and S. Kurinobu, Water Sci. Technol., 2004, 4, 47–54 Search PubMed.
- B. Liu, B. Y. Gao and X. Xu, Chem. Eng. J., 2011, 178, 232–238 CrossRef CAS.
- D. Liu, P. Wang, G. R. Wei, W. B. Dong and F. Hui, Environ. Sci. Pollut. Res., 2013, 20, 60–65 CrossRef CAS PubMed.
- R. R. Mohammed, M. R. Ketabchi and G. McKay, Chem. Eng. J., 2014, 243, 31–42 CrossRef CAS.
- J. Wang, J. Yang, H. W. Zhang, W. S. Guo and H. H. Ngo, J. Ind. Eng. Chem., 2015, 26, 37–45 CrossRef CAS.
- J. M. Oades, Soils Fert., 1963, 26, 69–80 CAS.
- P. J. Murphy, A. M. Posner and J. P. Quirk, Aust. J. Soil Res., 1975, 13, 189–201 CrossRef CAS.
- T. B. Zhen and T. H. Xiao, Environ. Chem., 1989, 8, 27–31 Search PubMed.
- H. Y. Dong, B. Y. Gao, Q. Y. Yue, H. Y. Rong, S. L. Sun and S. Zhao, Desalination, 2014, 335, 102–107 CrossRef CAS.
- H. Y. Dong, B. Y. Gao, Q. Y. Yue, Y. Wang and Q. Li, Chemosphere, 2015, 130, 90–97 CrossRef CAS PubMed.
- T. Li, Z. Zhu, D. S. Wang, C. H. Yao and H. X. Tang, Powder Technol., 2006, 168, 104–110 CrossRef CAS.
- P. Jarvis, B. Jefferson and S. A. Parsons, Environ. Sci. Technol., 2005, 39, 2307–2314 CrossRef CAS PubMed.
- W. Z. Yu, N. Graham, H. J. Liu and J. H. Qu, Chem. Eng. J., 2013, 234, 158–165 CrossRef CAS.
- S. G. Gu, K. J. Yan, S. C. Zhen and J. H. Wu, Environ. Prot. Chem. Ind., 2015, 35, 571–574 Search PubMed.
- H. Y. Dong, B. Y. Gao, Q. Y. Yue, S. L. Sun, Y. Wang and Q. Li, Chem. Eng. J., 2014, 258, 442–449 CrossRef CAS.
- B. Q. Zhao, D. S. Wang, T. Li, C. W. Chow and C. Huang, Sep. Purif. Technol., 2012, 72, 22–27 CrossRef.
- J. Wang, J. Guan, S. R. Santiwong and T. D. Waite, J. Membr. Sci., 2008, 321, 132–138 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |