DOI:
10.1039/C6RA19917H
(Paper)
RSC Adv., 2016,
6, 100765-100771
Effects of paclitaxel (PTX) prodrug-based self-assembly peptide hydrogels combined with suberoylanilide hydroxamic acid (SAHA) for PTX-resistant cancer and synergistic antitumor therapy†
Received
6th August 2016
, Accepted 13th October 2016
First published on 17th October 2016
Abstract
In this work, we designed a self-assembly peptide hydrogel encapsulated paclitaxel (PTX) and suberoylanilide hydroxamic acid (SAHA) for cancer co-delivery (Nap–PTX–SAHA). The hydrogels exhibited uniform nanofibers that entangle to form stable networks and pore microstructures (about 140–180 nm) resulting in absorption and storage of hydrophobic drugs. The circular dichroism (CD) spectra of hydrogels exhibit β-turn-like features. Nap–PTX–SAHA hydrogels achieved control over different types of drugs loading and released by diffusion and relaxation processes. Hydrogels exhibited better therapeutic effect and greater synergetic effect compared to the free drugs in vitro and in vivo. Injection of the hydrogels appears to achieve long-term drug release with higher efficacy and better biocompatibility than free drugs. As for the drug biodistribution studies in tumor-bearing mice, the PTX and SAHA from Nap–PTX–SAHA gels uptake in liver, lung and tumor were more than that of free PTX and SAHA. In comparison with free PTX or SAHA, the Nap–PTX–SAHA gels lower the uptake of released drug significantly in the heart, spleen, and kidney to reduce the cardiotoxicity and renaltoxicity. In conclusion, the Nap–PTX–SAHA gels certainly reduce the side effects of PTX and SAHA, enhance synergistic anticancer effects and suppress the drug resistance of PTX. This strategy can be a basis for designing appropriate clinical trials and will hold promise in nanomedicine for different drug combinations.
Introduction
Mortality by carcinoma has threatened millions of individual lives. Current chemotherapy cancer drugs often display lower biocompatibility, higher cytotoxicity and drug resistance.1–4 These may explain why so many of clinical trials have been disappointing. In recent years, several studies have been conducted to show that drug combination therapy could provide a promising strategy to suppress cancer-drug resistance.5 Different drugs may damage or kill cancer cells at different stages of their growth cycles. The multiple-drug payloads could display the ability to co-deliver multiple drugs, decreased toxicity, enhanced bioavailability, great accumulation at the tumors and prolonged plasma half-life.6–8 Cancer therapy has been improved significantly by combination chemotherapy performed with traditional anticancer drugs in clinical treatment. Paclitaxel (PTX) is one of the most extensively used cancer drugs in clinics. PTX shows promising results as an anti-cancer agent, stabilizing microtubules and preventing cell proliferation. Unfortunately, the use of PTX in cancer chemotherapy is often associated with a major obstacle of drug resistance. The drug resistance of PTX involves variations in tubulin structure, altered intracellular drug levels and signal transduction, evasion of apoptotic pathways and so on.9–11
Chemotherapy resistance is related to significant changes in gene expression. Therefore, epigenetic-mediated changes may be the responsible driving force for chemotherapy resistance.12,13 With the development of epigenetics research, histone deacetylase inhibitors (HDACIs) have become potential targets for chemotherapeutic intervention in cancer drug developments. Treatment of cancer with HDACIs has pleiotropic effects, inducing inhibition of HSP90, inhibiting cell proliferation, inducing cell-cycle arrest and apoptosis.14 Suberoylanilide hydroxamic acid (SAHA; Zolinza/vorinostat) is a HDACI that shows strong anticancer effects on various tumors. Moreover, SAHA is used in clinical trials for the treatment of solid and hematological tumors, which has particularly been FDA approved only for treatment of progressive or recurrent cutaneous T-cell lymphoma (CTCL).15,16 In addition, SAHA shows sensitive and synergistic effects with a variety of traditional chemotherapeutic drugs, such as decitabine and paclitaxel.17,18 Therefore, SAHA is a suitable candidate for combination therapy against chemotherapeutic resistance. Recently, several studies have demonstrated that SAHA could potentiate PTX-induced antitumor effects against some human cancer cells (lung cancer cells, ovarian cancer cells, endometrial cancer cells, etc.).19–22
More and more research studies concern designing nano-sized carriers for combination drug delivery, such as nanoparticles, liposomes, nanofibers and micelles.23–25 The prodrug-based delivery systems for drug combination are advanced and pioneering. Based on the previous researches of PTX-prodrug, we plan to simultaneously load PTX and SAHA into the same nano-carrier in the following three steps: (1) amphiphilic peptide series (Nap-FFE-CS-EYK) and self-assembled porous hydrogels (Nap-peptide) are designed; (2) conjugates of PTX and Nap-peptide are utilized to construct the prodrug-based delivery system (Nap-peptide–PTX); (3) Nap-peptide–PTX self-assembly hydrogels encapsulate SAHA through suitable porous nano-structure and non-covalent forces (Nap–PTX–SAHA). This system is expected to induce cell cycle arrest in a different phase of the cell cycle, reduce the side effects of PTX and SAHA, enhance synergistic anticancer effects and suppress the drug resistance of PTX.
Materials and methods
Chemicals and materials
Paclitaxel and SAHA were obtained commercially from Dalian Meilun Biotech Co. Ltd (Dalian, China); the N-Fmoc protected amino acids were obtained from GL Biochem Ltd. (Shanghai, china); 4-dimethylamino-pyridine (DMAP), 1-hydroxybenzotriazole (HOBt), trifluoroacetic acid (TFA), N,N-diisopropylethylamine (DIPA), O-benzotriazole-N,N,N′,N′-tetramethyl-uroniumhexafluoro-phosphate (TBTU), N,N′-diisopropylcarbodiimide (DIC), N-hydroxysuccinimide (NHS) and L-glutatthione (GSH) were purchased from Aladdin Reagent Corporation (Shanghai, China); Fmoc-OSu was purchased from J&K Scientific chemical Ltd. (Beijing, China); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Biosharp Company; indocyanine green (ICG) was obtained from Sangon Biotech Co. Ltd. (Shanghai, China); the H22 cells were purchased from American Type Culture Collection (ATCC, America); the heptoma ascites tumor mice model was a kind gift from Qinglong Guo group in China Pharmaceutical University; the BALB/c female mice were purchased from the Animal Care and Use Committee in China Pharmaceutical University. RPMI-1640 and fetal bovine serum (FBS) were obtained from Nanjing KeyGEN Biotech Corp. Ltd (Hyclone, America); all other reagents used in the study were analytical grade.
Characterization
Column chromatography was performed on silica gel (200–300 mesh). Thin layer chromatography (TLC) was performed using silica gel GF-254 pre-coated glass plates (0.25 mm) and analyzed by UV illumination. Hydrophilic products were purified with Agilent1200 (America) HPLC system. 1H NMR spectra were obtained on Bruker (Germany). LC-MS spectra were achieved on an Agilent 1260 Series HPLC system equipped with an API 4000 tandem mass spectrometer (America), containing a Turbo-V® ionspray source operated in the positive ESI mode. Rheology experiments were conducted on a Thermo RheoStress 600 instrument using a set of 60 mm diameter parallel plates with the thickness of 0.3 mm at 37 °C (Germany). The structures of peptide hydrogels were observed by Jasco-810 circular dichroism (Japan). TEM images of hydrogels solution with different concentration (1.0, 1.2, 1.4, and 1.6 wt%) were taken on a JEM-2100 transmission electron microscope (Japan). The sample powder of hydrogels after cryo-drying was deposited on a copper stub and the SEM images were taken by an S-4800 scanning electron microscope (Japan). Cytotoxicity tests were measured by BioRad431 microplate reader (America) and imaged on an Olympus IX71 confocal microscope (Japan).
Paclitaxel-succinyl-NHS active ester synthesis (PTX-succi-NHS)
The PTX-succi-NHS active ester was prepared according to previous ref. 26 with improvement: paclitaxel (0.78 g, 0.8 mmol) was added to succinic anhydride (0.28 g, 2.8 mmol) in the presence of DMAP (0.18 g, 1.4 mmol) which was previously dried under vacuum for 3 hours. Then 10 mL of dry pyridine was added and the solution was stirred for overnight at room temperature. The reaction mixture was extracted with dry dichloromethane (DCM) (20 mL × 3). Then the organic phase was washed using 1 M HCl (20 mL × 3) and water (20 mL × 3). The organic phase was combined and washed with brine (10 mL × 3) and dried over Na2SO4. The crude product was concentrated on a rotary evaporator and then dissolved in 5 mL of chloroform reacting with NHS (0.10 g, 0.8 mmol) and DIC (140 μL, 0.8 mmol) for overnight. The mixture was purified by using column chromatography (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DCM/methanol). The pure white product of PTX-succi-NHS was obtained with yield of 96.8%. TLC, Rf = 0.66 (DCM/MeOH, 10
1 DCM/methanol). The pure white product of PTX-succi-NHS was obtained with yield of 96.8%. TLC, Rf = 0.66 (DCM/MeOH, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). ESI-MS: C55H58N2O19, calc. MW = 1051.1, obsvd [M + Na]+ = 1073.4, [M + Na + H]+ = 1074.4. 1H NMR (300 MHz, CDCl3).
1). ESI-MS: C55H58N2O19, calc. MW = 1051.1, obsvd [M + Na]+ = 1073.4, [M + Na + H]+ = 1074.4. 1H NMR (300 MHz, CDCl3).
Fmoc-succinated cystamine synthesis (Fmoc-CS)
The pure white product of Fmoc-CS was synthesized by our group.27 The cystamine dihydrochloride (5 g, 22.2 mmol) and DIPA (7.7 mL, 44.4 mmol) were added into 25 mL of methanol with a vigorous stirring for 30 min in an ice-water bath. The succinic anhydride (2.22 g, 22.2 mmol) in 1,4-dioxane (50 mL) was added to the above solution within 60 min, and the solution was stirred for 15 min at room temperature. After removed solvent under vacuum the aqueous Na2CO3 solution (2.6 g, 25 mmol) was added, and then the solution was treated with ether (200 mL × 3) to remove unreacted materials and side products. The aqueous layer was collected then Na2CO3 (3.8 g, 36 mmol) was added. The solution was placed in an ice-water bath with a vigorous stirring to completely dissolute Na2CO3. Fmoc-OSu (7.4 g, 22.2 mmol) in 1,4-dioxane was added dropwise over 60 min and stirred at room temperature for overnight. The filtrate was obtained by filtration and then concentrated to remove the solvent. The aqueous layer was washed with 200 mL of ether and acidified with 1 M HCl to pH 1–2, and then extracted by DCM. The organic layer was dried over anhydrous Na2SO4 for 30 min and filtered. The solution was concentrated to a small volume and diluted with ether to give a white solid. The collected crystallized product was washed with ether, and dried in vacuum. The white solid powder of Fmoc-CS was obtained with yield of 51.8%. ESI-MS: C23H26N2O5S2, calc. MW = 474.6, obsvd [M + Na]+ = 497.1. 1H NMR (300 MHz, DMSO-d6).
Nap-FFE-CS-EYK synthesis (Nap-peptide)
The amino acid based hydrogel precursor was prepared by the standard solid-phase peptide synthesis (SPPS),28 which used 2-chlorotrityl chloride resin (1.0–1.2 mmol g−1) and Fmoc-amino acids. The Nap-peptide was obtained with yield of 89.2%. ESI-MS: C63H77N9O15S2, calc. MW = 1264.5, obsvd [M]+ = 1264.5, [M + H]+ = 1265.5. 1H NMR (300 MHz, DMSO-d6).
Nap-FFE-CS-EYK (paclitaxel) synthesis (Nap–PTX)
Nap-peptide (0.065 g, 0.045 mmol) and PTX-succi-NHS (0.0239 g, 0.025 mmol) were dissolved in DMF (2 mL), then DIPA (35 μL, 0.140 mmol) was added and stirred overnight in the dark. The product was purified by HPLC with yield of 78.4%. ESI-MS: C114H130N10O31S2, calc. MW = 2200.4, obsvd [M + Na]+ = 2223.1. 1H NMR (300 MHz, DMSO-d6).
Formation of Nap–PTX–SAHA hydrogels
Nap–PTX peptide was prepared at a final concentration of 1.5 mg mL−1 in PBS solution, and 6 equiv. of Na2CO3 (0.1 M) was used to adjust the pH value to about 7.4. The SAHA (1.0 mg mL−1) was then added to the solution while the formulation was stirred slowly. When completely dissolved the GSH solution (10 mg mL−1) was added to the solution to initiate hydrogelation. A transparent hydrogel formed within 10 minutes.
Release profile
In order to estimate the SAHA and PTX release profile of the drug delivery system, the Nap–PTX–SAHA hydrogels (1.5%) were immersed in 0.3 mL of phosphate buffer solution (PBS) at pH 7.4 and incubated at 37 °C. About 0.2 mL of the supernatant was removed at specific time intervals and the volume was reconstituted by adding 0.2 mL of fresh PBS each time. The test was repeated at least three times. Then, the sample solutions were analyzed to determine the amount of SAHA and PTX released by HPLC. The analysis was carried out on a reverse-phase column (Phenomenex C18 250 × 4.6 mm, 5 μm) using isocratic conditions (1 mL min−1) of 41% H2O (containing 0.1% phosphoric acid, pH 3), 36% acetonitrile and 23% methanol with UV detection at 227 nm. The concentration of drugs released in PBS was calculated using standard SAHA and PTX calibration curve and the drug release kinetics was studied. To further understand the release mechanism, the results were analyzed on the empirical eqn (1):29where Mt/M∞ is a fraction of drug released at time t, k is a release rate constant, and n is the diffusion exponent that gives an indication of the drug release mechanism. When n is less than 0.5, which is the Fickian diffusion (Higuchi model) mechanism, it occurs by the usual molecular diffusion of the drug due to a chemical potential gradient. When n is 1, it is associated with the relaxation release of a drug, leading to zero-order kinetics. When the n is between 0.5 and 1, anomalous transport is observed in which both Fickian diffusion and relaxation phenomena contribute to the drug release.
Cancer cell imaging
The plain ICG-hydrogels cells imaging probe were prepared using aqueous-based preparation method, in which Nap-peptide, Nap–PTX, Nap–SAHA and Nap–PTX–SAHA precursors were simply added to distilled water at room temperature. A stock solution of ICG was also placed in distilled water by mixing the ICG and hydrogels precursor according to the mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixed solutions were stirred at room temperature for 30 min. Solutions were filtered using 2000 Da filters (Millipore) to remove excess non-binding ICG. The formation of the hydrogels was according to the part “Formation of Nap–PTX–SAHA hydrogels” method. H22 (Murine hepatocellular carcinoma) cells were grown in RPMI 1640 (+) (L)-glutamine medium supplemented with 10% (v/v) fetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. The cells were maintained in an incubator with 5% CO2 at 37 °C. 24-Well plates were seeded with H22 cells per well in 100 μL of 3000 cells per mL medium overnight, and the cells were incubated with samples (Nap-peptide, Nap–PTX, Nap–SAHA and Nap–PTX–SAHA gels). After being cultured for another 12 h, cancer cells were observed using an optical microscope after being washed by PBS solution.
10. The mixed solutions were stirred at room temperature for 30 min. Solutions were filtered using 2000 Da filters (Millipore) to remove excess non-binding ICG. The formation of the hydrogels was according to the part “Formation of Nap–PTX–SAHA hydrogels” method. H22 (Murine hepatocellular carcinoma) cells were grown in RPMI 1640 (+) (L)-glutamine medium supplemented with 10% (v/v) fetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. The cells were maintained in an incubator with 5% CO2 at 37 °C. 24-Well plates were seeded with H22 cells per well in 100 μL of 3000 cells per mL medium overnight, and the cells were incubated with samples (Nap-peptide, Nap–PTX, Nap–SAHA and Nap–PTX–SAHA gels). After being cultured for another 12 h, cancer cells were observed using an optical microscope after being washed by PBS solution.
In vitro cytotoxicity study
100 μL of 2000 cells per mL H22 cells was seeded in each well on the 96-well plate overnight, and the cells were incubated with samples (Nap-peptide gels, PTX, SAHA, PTX + SAHA, Nap–PTX gels, Nap–SAHA gels, Nap–PTX gels + Nap–SAHA gels and Nap–PTX–SAHA gels) in a stepwise increasing concentration for 48 h. Control groups received an equal volume of PBS solution. The resistant cells were selected by removing the dead non-resistant cells. The above experiments were repeated 3 times. Data was expressed by cell survival. The number of viable cells was measured at 570 nm. The IC50 (the 50% inhibitory concentrations) and CI (combination index) were calculated using the equation below:| | |
CI = IC50 (PTX) pair/IC50 (PTX) + IC50 (SAHA) pair/IC50 (SAHA)
| (2) |
where IC50 (PTX) pair and IC50 (SAHA) pair are the half inhibitory concentrations when the drug is given as a PTX–SAHA pair; IC50 (PTX) and IC50 (SAHA) are the half inhibitory concentrations when the drug PTX or SAHA act singly. The CI values lower than, equal to, and higher than 1 indicate synergism, additivity and antagonism, respectively.
In vivo antitumor effect against drug resistant
This study was approved by the Ethics Committee of Central China Pharmaceutical University. All the in vivo experiments were conducted in accordance with the “Guidelines for the Care and Use of Laboratory Animals” published by the National Institute of Health (NIH Publication no. 85-23, revised 1985). The hepatoma H22 ascites tumor mice were cervical dislocation executed. The ascites tumor cells were extracted by sterilization syringe, diluted by saline, centrifuged with 1000 rpm for 5 min, and the supernatant was poured out. The above process was repeated 3 times. Every BALB/c mouse (4 weeks old, 22–24 g) was subcutaneously injected with 0.1 mL H22 cell suspension (106 cells per mL) at the right axillary region to establish the liver animal model. Tumor growth was monitored every other day and tumor volume (V) was calculated by the following formula (3). The relative tumor volume (RTV) was obtained from the ratio of the tumor volume at the different determination times and the original tumor volume (formula (3)). About 2 weeks later, 0.3–0.5 cm3 solid tumor growth was obviously exhibited in the mice. Tumor-bearing mice were randomly divided into 8 groups with 8 mice in each group and separately injected with 0.1 mL of saline, PTX (5 mg kg−1 and 10 mg kg−1), SAHA (5 mg kg−1 and 10 mg kg−1), Nap–PTX–SAHA hydrogels (10 mg kg−1, 20 mg kg−1 and 30 mg kg−1) at a single dose every other day. The first day of drug administration was day 0. Mice weight was monitored after receiving treatment and presented as relative weight (%).The number of long-term survivors and the survival time were detected. At the same time the diet, fur and activity of mice were observed.
Biodistribution in tumor-bearing mice
At the end of the two week, blood samples were taken by retro-orbital venous plexus puncture from tumor-bearing mice. The hearts, livers, spleens, kidneys, lungs and tumors of all the mice were immediately removed and washed with saline followed by homogenization with methanol or methyl tert-butyl ether for PTX and SAHA respectively. The mixture was treated with a vortex mixer for 3 min, followed by centrifugation with 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The PTX and SAHA concentration in the supernatant solution was detected by LC-MS quantitatively.
000 rpm for 5 min. The PTX and SAHA concentration in the supernatant solution was detected by LC-MS quantitatively.
Results and discussion
Nap–PTX–SAHA precursor formed a transparent hydrogel at the minimum gelation concentration (MGC) of 1.0 wt% in the phosphate buffer saline (PBS, pH 7.4) solution within 10 min at room temperature (22–25 °C). The mechanical property of resulting hydrogel (1.0 wt%) was characterized by a rheolometer to ascertain whether gels were injectable or not. After 24 h incubation, a PBS solution containing 1.0 wt% of Nap–PTX–SAHA in the rheolometer, the dynamic strain and frequency sweep were performed. As shown in Fig. 1A, the dynamic strain sweep indicates that the storage moduli (G′) of hydrogels are independent of strain until the critical strain is reached. The G′ values start to decrease drastically due to the breakdown of the networks of the hydrogels. After obtaining the maximum G′ values of the hydrogels in dynamic strain sweep, we measured the frequency dependence of hydrogels (Fig. 1B). The value of the G′ of gel is about 1200 Pa, and one magnitude bigger than that of its corresponding dynamic loss moduli (G′′) value (200 Pa). The result indicated the formation of a true gel which behaved as viscoelastic materials. The hydrogels were injectable and could be administered via subcutaneous or intratumor injection of animals.30
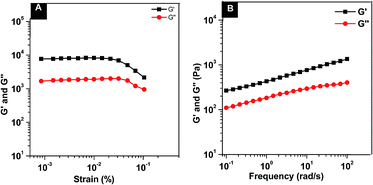 |
| | Fig. 1 Dynamic strain (A) and frequency sweep (B) of solutions containing 1.0 wt% of Nap–PTX–SAHA hydrogels. | |
The TEM images reveal ordered nanofibers on the morphology of self-assembled structures. As shown in Fig. 2, the TEM images of all the hydrogels, which consist of different concentrations, exhibit long, flexible, and uniform nanofibers that entangle to form stable networks. With the increase of the concentrations of hydrogelator (1.0, 1.2, 1.4, and 1.6 wt%), the densities of the nanofibers in the hydrogels increase, but the widths of the nanofibers in the hydrogels remain similar (around 17 ± 2 nm). These results indicate that the nanofibers exhibit similar morphology regardless of the concentrations of the precursor solutions. These nanofibers entangle each other to form three-dimensional networks to support the hydrogels formation. In addition, the SEM images (Fig. 3) of hydrogels show that self-assembled peptides could form micropores (about 140–180 nm), thus resulting in absorption and storage of hydrophobic drugs.
 |
| | Fig. 2 TEM images of Nap–PTX–SAHA hydrogels solution with concentrations of 1.0 wt%, 1.2 wt%, 1.4 wt% and 1.6 wt% (scale bar is: 0.5 μm). | |
 |
| | Fig. 3 SEM images of the Nap–PTX–SAHA hydrogels after cryo-drying ((A) scale bar: 2 μm, Mag. = 20.00k×; (B) scale bar: 1 μm, Mag. = 40.00k×). | |
CD spectra of Nap–PTX gels and Nap–PTX–SAHA gels solution (0.1 mg mL−1) further help elucidate the molecular arrangement of gels phase (Fig. 4A). The spectrum of Nap–PTX gels exhibits a positive band near 207 nm and a broad positive band near 229 nm, indicating the existence of β-turn-like features. Moreover, comparing the CD spectra of Nap–PTX gels, Nap–PTX–SAHA gels also have β-turn-like molecular arrangement indicated by the similar peaks at 206 nm and 228 nm. Collectively, TEM, SEM and CD indicate that Nap–PTX–SAHA gels self-assemble into a β-turn-like structure to produce nanofibers that reach high density and result in β-turn-like matrices in the hydrogels. The Nap–PTX–SAHA hydrogels (1.5 wt%) exhibit two stage release profiles for PTX and SAHA respectively (Fig. 4B). The hydrogels slowly release PTX at a rate of 8.40 ± 0.02 μg per mL per hour within 12 hours. The SAHA was released at a rate of 326.4 ± 2.20 μg per mL per hour in the 7 hours, followed by a rate of 30.4 ± 2.20 μg per mL per hour in the next 5 hours, respectively. The release mechanism was analyzed on the basis of the empirical equation, which yields a convenient measure of release rate in the value of diffusion exponent (n).31 The n value of PTX is about 1 (n = 1.001, r = 0.9982), which is associated with the relaxation release of drug leading to zero-order kinetics. The n value of SAHA is between 0.5 and 1 (n = 0.5493, r = 0.9708), indicating the anomalous nature of drug release, to which both diffusion and relaxation processes contribute. The above results provide the recognition that the drug co-delivery hydrogels achieved the control over different types of drug loading and released by different processes, therefor achieving a better effect of synergy.32
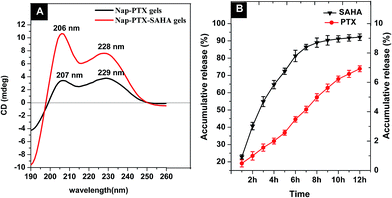 |
| | Fig. 4 (A) The CD images of the Nap–PTX gels and Nap–PTX–SAHA gels solution (0.1 mg mL−1); (B) accumulative release profile of PTX and SAHA from hydrogels at 37 °C in 0.1 M PBS solutions (pH 7.4, n = 3). | |
The cancer cells were incubated with Nap-peptide, Nap–PTX, Nap–SAHA and Nap–PTX–SAHA gels for 12 h to characterize drug synergistic anticancer effect. The viability of cells treated with the Nap-peptide gels shows no apparent change (Fig. 5A), indicating the carrier is hardly cytotoxic. The cells with Nap–PTX, Nap–SAHA gels are bloated and abnormal (Fig. 5B and C), even drastic cells morphologic change is observed with the Nap–PTX–SAHA gels evidences (Fig. 5D). These images correlated well with the results of MTT assay, further to be studied. The cytotoxicity of samples was determined by MTT assay against H22 cells. The results show no apparent cytotoxicity of Nap-peptide hydrogels at tested concentrations (Fig. 6). Table 1 shows the IC50 and combination indexes (CI) of the different therapeutic formulations. The IC50 values of all drug combinations with different formulations are lower than the single drug groups. The lowest IC50 value of 9.39 ± 0.024 nM and CI value of 0.487 are detected in the Nap–PTX–SAHA gels. It is found that the Nap–PTX–SAHA gels displayed the best anticancer effect and the greatest synergy in all formulations.
 |
| | Fig. 5 Confocal images of H22 cells after incubation with Nap-peptide (A), Nap–PTX (B), Nap–SAHA (C) and Nap–PTX–SAHA (D) hydrogels for 12 h after being washed by PBS solution (bar: 50 μm). | |
 |
| | Fig. 6 Cytotoxicity of samples (Nap-peptide gels, PTX, SAHA, PTX + SAHA, Nap–PTX gels, Nap–SAHA gels, Nap–PTX + Nap–SAHA gels and Nap–PTX–SAHA gels) after incubated with H22 cells. | |
Table 1 IC50 values of different formulations in drug resistant H22 cellsa
| Formulation |
IC50 (nM) |
CI |
| Data were given as mean ± SD (P < 0.05). |
| PTX |
25.04 ± 0.035 |
|
| SAHA |
14.96 ± 0.027 |
|
PTX + SAHA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
11.28 ± 0.019 |
0.602 |
| Nap–PTX gels |
27.06 ± 0.028 |
|
| Nap–SAHA gels |
14.23 ± 0.039 |
|
Nap–PTX + Nap–SAHA gels (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
11.70 ± 0.029 |
0.627 |
Nap–PTX–SAHA gels (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
9.39 ± 0.024 |
0.487 |
In order to determine the effect of free drugs and co-delivery, in vivo anti-tumor activity was examined in BALB/c mice implanted with H22 cells. As shown in Fig. 7A, the final relative tumor volumes are 1151.5%, 976.3%, 1246.9%,1192.5% and 1477.2% in groups administered with PTX (5 mg kg−1 and 10 mg kg−1), SAHA (5 mg kg−1 and 10 mg kg−1) and PBS solution, respectively. However, the final relative tumor volumes of Nap–PTX–SAHA gels (10 mg kg−1, 20 mg kg−1 and 30 mg kg−1) are about 564.7%, 437.7% and 379.4% far smaller than those of the control group after day 12. These observations clearly indicate that our hydrogels of PTX and SAHA could efficiently reduce the relative tumor sizes and hinder the growth of hepatoma more significantly than free drugs. The results indicated that Nap–PTX–SAHA gels displayed higher anticancer and synergy effect than free drugs, which corresponded to the results of the in vitro cytotoxicity investigation. Fig. 7B shows that the relative weights of mice injected with PTX, SAHA and the PBS control groups always shows an increasing trend, which is similar to their relative tumor volumes. It may be due to the tumor growth. The relative weight of animals in the group injected with hydrogels is relatively stable.
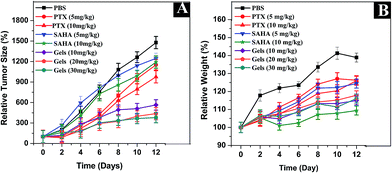 |
| | Fig. 7 Relative tumor size (A) and relative weights (B) of mice treated with PBS, PTX (5 mg kg−1 and 10 mg kg−1), SAHA (5 mg kg−1, 10 mg kg−1) and Nap–PTX–SAHA gels (10 mg kg−1, 20 mg kg−1 and 30 mg kg−1). | |
During the 12 days treatment, the mice groups administered with PTX or SAHA showed hair loss, skin chapping on the tumor site, even peeling, and there was a death in the PTX group (10 mg kg−1) at day 10 (Fig. 8A). In contrast, in the group administered with Nap–PTX–SAHA gels no obvious abnormality was observed in their activities, eating behavior and weight. The above results suggest that Nap–PTX–SAHA gels certainly limited the side-effects and enhanced the anticancer effect of free drugs in the mice (Fig. 8B). The injection of the hydrogels appears to achieve long-term drug release with higher efficacy and better biocompatibility than free drugs.
 |
| | Fig. 8 The tumor apparent (A) and tumor size (B) of mice treated with PBS, PTX (5 mg kg−1 and 10 mg kg−1), SAHA (5 mg kg−1, 10 mg kg−1) and Nap–PTX–SAHA hydrogels (10 mg kg−1, 20 mg kg−1 and 30 mg kg−1). | |
The biodistribution of free drugs and drug co-delivery systems was investigated in tumor xenograft mice. As shown in Fig. 9, in plasma, the total concentration of PTX and SAHA released from Nap–PTX–SAHA gels are much higher than that of free PTX and SAHA. It is also displayed that the free PTX and SAHA are all eliminated quickly from systemic circulation. The Nap–PTX–SAHA gels enhance the retention of PTX and SAHA in the circulation due to the self-assembly, which equipped the gels with the hydrophilic coat to keep the long term retention in the systemic circulation. As for the drug biodistribution in tumor-bearing mice, the PTX and SAHA from Nap–PTX–SAHA gels uptaked in liver, lung and tumor were more than that of free PTX and SAHA. The results are beneficial to these tissues in tumor treatment. In comparison with free PTX or SAHA, the Nap–PTX–SAHA gels lower the uptake of released drug significantly in heart, spleen, and kidney. This approach of co-delivery of drugs reduces the cardiotoxicity and renaltoxicity of PTX and SAHA. These are the indications of the improvement in the safety and therapeutic efficiency of drug co-delivery to reverse drug resistance.
 |
| | Fig. 9 Drug biodistribution in tumor-bearing mice (n = 8 per group, equivalent dose of drug = 10 mg kg−1). Data were given as mean ± SD (p < 0.05). | |
Conclusions
In conclusion, our study provides a novel peptide hydrogel which encapsulates PTX and SAHA in the same co-delivery nano-carrier to reverse drug resistance. The Nap–PTX–SAHA gels proposed best therapeutic effect and greatest synergetic effect compared to the free drugs in vitro and in vivo. The SAHA is able to prevent or reverse PTX resistance in cancer therapy. Since the strategy will be the basis for designing appropriate clinical trials, it will hold promise in nanomedicine for different drug combinations.
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (No. 81603072) and (No. 81573387).
Notes and references
- D. Das, P. Ghosh, A. Ghosh, C. Haldar, S. Dhara, A. B. Panda and S. Pal, ACS Appl. Mater. Interfaces, 2015, 7, 14338–14351 CAS.
- S. Fusco, H.-W. Huang, K. E. Peyer, C. Peters, M. Häberli, A. Ulbers, A. Spyrogianni, E. Pellicer, J. Sort, S. E. Pratsinis, B. J. Nelson, M. S. Sakar and S. Pané, ACS Appl. Mater. Interfaces, 2015, 7, 6803–6811 CAS.
- B. S. Pattni, V. V. Chupin and V. P. Torchilin, Chem. Rev., 2015, 115, 10938–10966 CrossRef CAS PubMed.
- X. Yuan, D. C. Marcano, C. S. Shin, X. Hua, L. C. Isenhart, S. C. Pflugfelder and G. Acharya, ACS Nano, 2015, 9, 1749–1758 CrossRef CAS PubMed.
- P. T. Wong and S. K. Choi, Chem. Rev., 2015, 115, 3388–3432 CrossRef CAS PubMed.
- O. Cohen and R. Granek, Nano Lett., 2014, 14, 2515–2521 CrossRef CAS PubMed.
- J. Han, T. Lei and Q. Wu, Carbohyd. Polym., 2014, 102, 306–316 CrossRef CAS PubMed.
- E. Ezan, Adv. Drug Delivery Rev., 2013, 65, 1065–1073 CrossRef CAS PubMed.
- Q. Yang, K. Wang, J. Nie, B. Du and G. Tang, Biomacromolecules, 2014, 15, 2285–2293 CrossRef CAS PubMed.
- R. Negrini, A. Sánchez-Ferrer and R. Mezzenga, Langmuir, 2014, 30, 4280–4288 CrossRef CAS PubMed.
- C. Yang, D. Li, Q. FengZhao, L. Wang, L. Wang and Z. Yang, Org. Biomol. Chem., 2013, 11, 6946–6951 CAS.
- D. M. Fass, S. A. Reis, B. Ghosh, K. M. Hennig, N. F. Joseph, W.-N. Zhao, T. J. F. Nieland, J.-S. Guan, C. E. Groves Kuhnle, W. Tang, D. D. Barker, R. Mazitschek, S. L. Schreiber, L.-H. Tsai and S. J. Haggarty, Neuropharmacology, 2013, 64, 81–96 CrossRef CAS PubMed.
- S. Mueller, X. Yang, T. L. Sottero, A. Gragg, G. Prasad, M.-Y. Polley, W. A. Weiss, K. K. Matthay, A. M. Davidoff, S. G. DuBois and D. A. Haas-Kogan, Cancer Lett., 2011, 306, 223–229 CrossRef CAS PubMed.
- L. Liu, J.-C. Detering, T. Milde, W. E. Haefeli, O. Witt and J. Burhenne, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 964, 212–221 CrossRef CAS PubMed.
- B. Munteanu, B. Meyer, C. von Reitzenstein, E. Burgermeister, S. Bog, A. Pahl, M. P. Ebert and C. Hopf, Anal. Chem., 2014, 86, 4642–4647 CrossRef CAS PubMed.
- C.-C. Yu, S.-L. Pan, S.-W. Chao, S.-P. Liu, J.-L. Hsu, Y.-C. Yang, T.-K. Li, W.-J. Huang and J.-H. Guh, Biochem. Pharmacol., 2014, 90, 320–330 CrossRef CAS PubMed.
- V. Brabec, D. M. Griffith, A. Kisova, H. Kostrhunova, L. Zerzankova, C. J. Marmion and J. Kasparkova, Mol. Pharm., 2012, 9, 1990–1999 CrossRef CAS PubMed.
- B. E. Gryder, M. J. Akbashev, M. K. Rood, E. D. Raftery, W. M. Meyers, P. Dillard, S. Khan and A. K. Oyelere, ACS Chem. Biol., 2013, 8, 2550–2560 CrossRef CAS PubMed.
- A. Angelucci, M. Mari, D. Millimaggi, I. Giusti, G. Carta, M. Bologna and V. Dolo, Gynecol. Oncol., 2010, 119, 557–563 CrossRef CAS PubMed.
- A. G. Assanhou, W. Li, L. Zhang, L. Xue, L. Kong, H. Sun, R. Mo and C. Zhang, Biomaterials, 2015, 73, 284–295 CrossRef CAS PubMed.
- C. S. Dietrich Iii, V. L. Greenberg, C. P. DeSimone, S. C. Modesitt, J. R. van Nagell, R. Craven and S. G. Zimmer, Gynecol. Oncol., 2010, 116, 126–130 CrossRef CAS PubMed.
- V. Zuco, M. De Cesare, R. Cincinelli, R. Nannei, C. Pisano, N. Zaffaroni and F. Zunino, PLoS One, 2011, 6, e29085 CAS.
- D. Caccavo, S. Cascone, G. Lamberti and A. A. Barba, Mol. Pharm., 2015, 12, 474–483 CrossRef CAS PubMed.
- E. M. Cahill and E. D. O'Cearbhaill, Bioconjugate Chem., 2015, 26, 1289–1296 CrossRef CAS PubMed.
- D. Ma, S. Tian, J. Baryza, J. C. Luft and J. M. DeSimone, Mol. Pharm., 2015, 12, 3518–3526 CrossRef CAS PubMed.
- Y. Gao, Y. Kuang, Z.-F. Guo, Z. Guo, I. J. Krauss and B. Xu, J. Am. Chem. Soc., 2009, 131, 13576–13577 CrossRef CAS PubMed.
- C. Shu, R. Li, Y. Yin, D. Yin, Y. Gu, L. Ding and W. Zhong, Chem. Commun., 2014, 50, 15423–15426 RSC.
- H. Wang, L. Lv, G. Xu, C. Yang, J. Sun and Z. Yang, J. Mater. Chem., 2012, 22, 16933–16938 RSC.
- P. L. P. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 23–26 CrossRef CAS.
- W. Zheng, J. Gao, L. Song, C. Chen, D. Guan, Z. Wang, Z. Li, D. Kong and Z. Yang, J. Am. Chem. Soc., 2012, 135, 266–271 CrossRef PubMed.
- H. A. Kim, K. Nam and S. W. Kim, Biomaterials, 2014, 35, 7543–7552 CrossRef CAS PubMed.
- E. Nazaruk, M. Szlęzak, E. Górecka, R. Bilewicz, Y. M. Osornio, P. Uebelhart and E. M. Landau, Langmuir, 2014, 30, 1383–1390 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization. See DOI: 10.1039/c6ra19917h |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DCM/methanol). The pure white product of PTX-succi-NHS was obtained with yield of 96.8%. TLC, Rf = 0.66 (DCM/MeOH, 10
1 DCM/methanol). The pure white product of PTX-succi-NHS was obtained with yield of 96.8%. TLC, Rf = 0.66 (DCM/MeOH, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). ESI-MS: C55H58N2O19, calc. MW = 1051.1, obsvd [M + Na]+ = 1073.4, [M + Na + H]+ = 1074.4. 1H NMR (300 MHz, CDCl3).
1). ESI-MS: C55H58N2O19, calc. MW = 1051.1, obsvd [M + Na]+ = 1073.4, [M + Na + H]+ = 1074.4. 1H NMR (300 MHz, CDCl3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixed solutions were stirred at room temperature for 30 min. Solutions were filtered using 2000 Da filters (Millipore) to remove excess non-binding ICG. The formation of the hydrogels was according to the part “Formation of Nap–PTX–SAHA hydrogels” method. H22 (Murine hepatocellular carcinoma) cells were grown in RPMI 1640 (+) (L)-glutamine medium supplemented with 10% (v/v) fetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. The cells were maintained in an incubator with 5% CO2 at 37 °C. 24-Well plates were seeded with H22 cells per well in 100 μL of 3000 cells per mL medium overnight, and the cells were incubated with samples (Nap-peptide, Nap–PTX, Nap–SAHA and Nap–PTX–SAHA gels). After being cultured for another 12 h, cancer cells were observed using an optical microscope after being washed by PBS solution.
10. The mixed solutions were stirred at room temperature for 30 min. Solutions were filtered using 2000 Da filters (Millipore) to remove excess non-binding ICG. The formation of the hydrogels was according to the part “Formation of Nap–PTX–SAHA hydrogels” method. H22 (Murine hepatocellular carcinoma) cells were grown in RPMI 1640 (+) (L)-glutamine medium supplemented with 10% (v/v) fetal bovine serum, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin. The cells were maintained in an incubator with 5% CO2 at 37 °C. 24-Well plates were seeded with H22 cells per well in 100 μL of 3000 cells per mL medium overnight, and the cells were incubated with samples (Nap-peptide, Nap–PTX, Nap–SAHA and Nap–PTX–SAHA gels). After being cultured for another 12 h, cancer cells were observed using an optical microscope after being washed by PBS solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The PTX and SAHA concentration in the supernatant solution was detected by LC-MS quantitatively.
000 rpm for 5 min. The PTX and SAHA concentration in the supernatant solution was detected by LC-MS quantitatively.





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)



