Sustained release of nerve growth factor from highly homogenous cubosomes stabilized by β-casein with enhanced bioactivity and bioavailability
Abstract
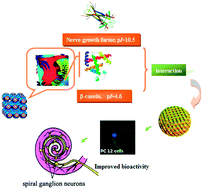
Controlling the specific ionic interactions between drugs and mesophases particles can enable controlled release systems for proteins. Here we present the interaction of basic nerve growth factor (NGF) with highly homogeneous cubosomes based on the glycerol monooleate–water system where β-casein was employed as a stabilizer. Addition of β-casein leads to modification of the internal cubosomal surface to generate highly negative charge. The colloidal structures were characterized with small angle X-ray scattering and visualized using cryogenic transmission electron microscopy. These nanostructured particles are able to sustain the release of hydrophilic NGF since less than 15% of the protein was released over 24 h. Similarly, cationic 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) immobilized within the mesophase particles provided another specific interactions of the cubosomal surface, from which the release of water soluble bovine serum albumin was retarded. Evaluation of NGF entrapped colloidal dispersions in PC12 cells led to identification of β-casein stabilized cubosomes that induce more than 140% neurite outgrowth enhancing the activity of NGF. Accordingly, the AUC value for RWM administration of NGF-loaded dispersions in guinea pigs was significantly higher than that administrated with NGF, of which the relative bioavailability of the β-casein stabilized dispersions was increased to 337% as compared with free drug.

 Please wait while we load your content...
Please wait while we load your content...