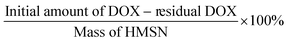Constructing H+-triggered bubble generating nano-drug delivery systems using bicarbonate and carbonate†
Zuhuang Wena,
Yijuan Longa,
Lili Yanga,
Jiangang Hub,
Ning Huangb,
Yuan Chengb,
Li Zhaoc and
Huzhi Zheng*a
aKey Laboratory on Luminescent and Real-Time Analytical Chemistry (Southwest University), College of Chemistry and Chemical Engineering, Southwest University, Beibei, Chongqing, 400715, China. E-mail: zhenghz@swu.edu.cn
bDepartment of Neurosurgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, P. R. China
cSouthwest University Hospital, Southwest University, Beibei, Chongqing, 400715, China
First published on 25th October 2016
Abstract
Nanoparticles have the great potential to act as drug delivery carriers, and such systems must effectively deliver the drug to the cancer cells, and provide intracellular drug release. In this research, two types of H+-triggered bubble generating nanosystems (BGNS) were developed which were obtained using hollow mesoporous silica nanoparticles loaded with doxorubicin and subsequently treated with bubble generating agents, i.e., sodium bicarbonate (SBC) and ammonium carbonate (AC). These were named BGNS-SBC and BGNS-AC. After internalization by the tumor cells, the H+ reacts with bicarbonate or carbonate to generate carbon dioxide bubbles quickly in an acidic environment of endo/lysosomes, which results in lysosomal membrane permeabilization (LMP). Then, the enhanced LMP induced cancer cell death by an apoptosis-like pathway because of the release of caspase-3 from the lysosome into the cytoplasm. In addition, BGNS-SBC and BGNS-AC possessed remarkable cytotoxicity against MCF-7 cells and efficiently overcame the multidrug resistance (MDR) of MCF-7/adriamycin cells. Therefore, these bubble generating nanosystems could be a promising nanocarrier for the treatment of tumors.
1 Introduction
Although great progress has been made in developing validated treatments for different forms of cancer, multidrug resistance (MDR) to chemotherapeutic agents is still a challenge to effective therapy for cancer,1,2 and this is mainly because of the over expression of P-glycoprotein (P-gp) membrane proteins which mediate drug efflux in cancer cells.3,4 In order to solve this difficult challenge, diverse cancer therapies have been utilized, such as photodynamic therapy,5 nuclear-targeted drug delivery systems,6 short interfering RNAs for cancer gene therapy,7 bacterial8 and viral-based therapy9 and these have been exploited to overcome the MDR of cancer cells. In particular, a number of nanocarrier-based drug delivery systems have been approved for clinical applications in solid tumors.10,11 With the enhanced permeability and retention effect, nanocarriers can efficiently improve cellular uptake, stability and accumulation of drugs at disease sites without leakage and provide intracellular drug release to achieve an enhanced intracellular drug concentration that would cause the cells' death.Lysosomes have been perceived as intracellular “suicide bags”,12 once the lysosome membrane integrity has been impaired. The release of certain cathepsins from the lysosome into the cytoplasm is considered to trigger pyroptosis or necrosis.13,14 Therefore, these organelles have attracted considerable interest as potential therapeutic targets in cancer.15,16
Because of the biosafety issues with using drugs in the clinic environment, it is desirable to use a lower amount of drug nanocarriers with a higher drug loading capability for drug delivery applications.17 Hollow mesoporous silica nanoparticles (HMSN) have been developed and gained increasing attention because of their large surface area, high pore volume, excellent biocompatibility, and large hollow interiors for the storage of more drugs.18 As one of the potent anticancer drugs, doxorubicin (DOX) has been used clinically to treat a wide range of cancers including breast cancer, lung cancer, ovarian cancer, and soft tissue sarcoma, because it has available advantages of efficacy and price.19 As well as these therapeutic applications, the intrinsic fluorescence of DOX also means that it has a role as a fluorescent probe.20 Consequently, there is extensive interest in using DOX as a multifunctional drug model for cancer therapy.21 However, DOX still possesses serious limitations such as undesired systemic toxicity resulting from the lack of tumor selectivity and anti-tumor efficacy.22 To overcome these defects, considerable efforts have been devoted to encapsulating DOX into various nanocarriers, including liposomes, micelles, and mesoporous silica nanoparticles.23
Sodium bicarbonate (SBC; NaHCO3) was found to be a bubble generating agent when it was used in acidic conditions. Reaction of SBC with an acid produces a carbonic acid, which readily decomposes to carbon dioxide (CO2) bubbles and water (H2O).24 Ke et al. used SBC to construct CO2 bubble generating nanocarriers to accelerate the release of DOX.25,26 Additionally, DOX reacts with SBC to produce a red precipitate which could be dissolved in an acidic phosphate buffer solution and then used to generate bubbles as described by Yang et al.27 In this research, two types of H+-triggered bubble generating drug delivery nanosystems (BGNS) were constructed, which were prepared with HMSN loaded with DOX and subsequently treated with bubble generating agents, i.e., SBC or ammonium carbonate (AC; (NH4)2CO3). Scheme 1 is a schematic illustration of the BGNS and how they work. Subsequently, in vitro drug release behavior, lysosomal membrane permeabilization (LMP) studies, and caspase-3 levels in MCF-7 breast cancer cells were extensively evaluated. Two types of drug delivery systems, were demonstrated, both of which are highly promising for use in simultaneous cancer therapy as well as for overcoming drug tolerance.
 | ||
| Scheme 1 Schematic illustration of the formation of BGNS and showing how they kill cancer cells using the quick release of CO2 bubbles. | ||
2 Experimental section
2.1 Materials
Trimethoxy(octadecyl)silane (C18TMS), and doxorubicin hydrochloride were purchased from Aladdin Reagents (Shanghai, China). Tetraethyl orthosilicate (TEOS) was acquired from Sigma-Aldrich (St. Louis, MO, USA). The MCF-7 cell line was purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China), and the MCF-7/adriamycin (ADR) cell line was purchased from ATCC (Shanghai, China). The cell medium and antibiotics were purchased from Gibco (Burlington, ON, Canada). The Cell-Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies (Kumamoto, Japan). Alexa Fluor® 488 dextran (molecular weight = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from ThermoFisher Scientific Inc. (MA, USA). The Caspase-3 Activity Assay Kit was obtained from the Beyotime Institute of Biotechnology (Nantong, China). All other reagents and solvents were of analytical grade and used without further purification. Ultrapure water (18.2 MΩ) was prepared with a Milli-Q® water purification system (Merck Millipore, USA) and used in all experiments.
000) was purchased from ThermoFisher Scientific Inc. (MA, USA). The Caspase-3 Activity Assay Kit was obtained from the Beyotime Institute of Biotechnology (Nantong, China). All other reagents and solvents were of analytical grade and used without further purification. Ultrapure water (18.2 MΩ) was prepared with a Milli-Q® water purification system (Merck Millipore, USA) and used in all experiments.
2.2 Synthesis of HMSN
HMSN were prepared according to a method described in a previous report.28 Briefly, ethanol (71.4 mL), H2O (10 mL), and ammonium solution (3.14 mL) were mixed and stirred at 30 °C for 1 h. Then, TEOS (6 mL) was added into the mixture, and the reaction was continued for another 1 h. TEOS (5 mL) and C18TMS (3 mL) were premixed and added rapidly into the reaction medium afterwards, and the reaction was allowed to continue for another 1 h. The nanoparticles were collected and washed three times with water. Then, the nanoparticles obtained were dispersed into an aqueous solution of sodium carbonate (0.2 M, 100 mL) for 4 h at 80 °C. The product was collected by centrifugation at 8000 rpm for 10 min and washed six times with water and then dried under vacuum overnight at 40 °C. Finally, the HMSN were obtained using calcination at 600 °C for 6 h.2.3 Preparation and characterization of BGNS
HMSN (80 mg) was dispersed into 10 mL of an aqueous solution of DOX (1 mg mL−1). After stirring in the dark for 24 h, the DOX-loaded HMSN (DMSN) were obtained. Then, the DMSN were dispersed into 12 mL of 0.2 M SBC solution or AC solution, and the mixtures were stirred under darkness for 12 h. The precipitates were collected by centrifugation at 8000 rpm for 10 min and washed three times with water. After dispersion in water, the resulting products were kept below 4 °C, and were named BGNS-SBC (treated with SBC) or BGNS-AC (treated with AC). To determine the drug loading content, the ultraviolet-visible (UV/Vis) absorbance of the supernatant containing DOX extracted from the drug delivery system was measured at 480 nm using a UV-Vis spectrophotometer (UV-2450, Shimadzu, Japan). The loading efficiency of DOX was calculated using the following equation:The morphology and mesostructure of HMSN, BGNS-SBC and BGNS-AC were observed using a transmission electron microscope (TEM; JEM-1200EX, Jeol Technics, Tokyo, Japan) with an accelerating voltage of 200 kV.
2.4 In vitro release experiment
To measure in vitro drug release, 100 μL of BGNS-SBC or BGNS-AC solution (500 μg mL−1, concentration in DOX) was added into 48-well plates that contained 900 μL of phosphate buffered saline (PBS) with different pH values (7.4, 5.0), respectively. The 48-well plate was gently shaken in a thermostatic rotary shaker at 250 rpm at 37 °C in the dark. For the measurement of released DOX concentration, the absorbance of the release medium at 480 nm was recorded using a UV-Vis spectrophotometer. Finally, after incubation at different pH conditions for 48 h, the morphology of BGNS-SBC and BGNS-AC were measured using TEM. The generation of CO2 bubbles from BGNS-SBC or BGNS-AC at different pH conditions was visualized using an ultrasound imaging system with a 7 MHz transducer (HD 11XE, Philips, Holland).2.5 Cell culture
Cells were incubated in Dulbecco's Modified Eagle's Medium (DMEM; MCF-7) or Roswell Park Memorial Institute (RPMI) 1640 medium (MCF-7/ADR) supplemented with 10% fetal bovine serum (Gicbo), 100 U mL−1 of penicillin and 100 μg mL−1 streptomycin and the cells were maintained at 37 °C in a humidified 5% CO2 incubator. The culture media was changed every two days. To maintain the resistant phenotype, MCF-7/ADR cells were maintained in the medium containing 1.0 μg mL−1 DOX and were cultured in a drug free medium for 48 h before the experiments.2.6 Confocal laser-scanning microscopy (CLSM)
MCF-7 cells were seeded at a density of 1 × 105 cells per well in 35 mm glass bottomed culture dishes (NEST Science Co, Ltd, China) and incubated for 24 h at 37 °C under 5% CO2. Then, free DOX, BGNS-SBC and BGNS-AC at the same drug concentration of 2.0 μg mL−1 were added to the contents of the culture dish. After incubation for different times (4, 12 or 24 h), the cells were washed with three times with PBS, followed by nuclei staining using Hoechst 33342 stain for 1 h and then stained with LysoTracker® Green (lysosome dye) for another 30 min. Finally, the culture dishes were visualized under a CLSM (TCS SP5, Leica Microsystems, Germany). The fluorescence images were taken under a 60× oil-immersion objective [numerical aperture (NA) = 1.42].2.7 In vitro cytotoxicity studies
After treatment with 0.25% trypsin, MCF-7 or MCF-7/ADR cells were harvested and seeded in 96-well plates at a density of 8000 cells per well and were incubated in 5% CO2 at 37 °C for 24 h. The growth media were replaced by fresh complete medium containing HMSN, free DOX, BGNS-SBC or BGNS-AC at a series of concentrations and incubated for 24 or 48 h. The, cell viability was then evaluated using the CCK-8 assay. Each data point is represented as mean ± standard deviation (SD) of the three independent experiments. In each experiment, all the drug concentrations were tested with six replicates.2.8 Visualization of LMP
To measure LMP, MCF-7 cells were seeded in 35 mm glass bottomed culture dishes at a density of 1 × 105 cells per dish and incubated for 24 h at 37 °C under 5% CO2 and incubated with 100 μg mL−1 Alexa Fluor 488 dextran for 1 h. Then, the cells were washed with three times with PBS. After the culture media were replaced by fresh medium containing free DOX, BGNS-SBC or BGNS-AC at a DOX concentration of 2 μg mL−1, the cells were incubated for another 4 h. The fluorescence images were collected on a fluorescence microscope (IX70, Olympus) equipped with a 100× oil immersion objective (NA = 1.30) and a blue illumination filter set (450–480/500/525/39 nm). Finally, the percentage of cells with LMP was obtained by counting randomly chosen areas, with a minimum of 100 cells for each experimental condition. Each data point is represented as mean ± SD of three independent experiments.2.9 Caspase-3 activity level
A commercial Caspase-3 Activity Assay Kit was used to determine caspase-3 activity. First, MCF-7 cells were seeded in 6-well plates at a density of 1 × 105 cells per well. After incubation for 24 h, cells were washed three times with PBS and the culture media was replaced by fresh culture media containing free DOX, BGNS-SBC or BGNS-AC at a DOX concentration of 2 μg mL−1, and then the cells were incubated for another 48 h at 37 °C under 5% CO2. Then, the cells were treated with pyrolysis liquid and centrifuged to obtain a sample for protein analysis. After incubating with reaction buffer and caspase-3 substrate at 37 °C for 4 h, the caspase-3 activity was determined at 405 nm using a microplate reader (Infinite 200 PRO, Tecan, Austria).2.10 Statistical analysis
Data were described as the mean ± SD, and statistical analysis was performed using a one-way analysis of variance (ANOVA). Statistical significance was set at *p < 0.05, and extreme significance was set at **p < 0.01 and ***p < 0.001.3 Results and discussion
3.1 Characterization of HMSN
HMSN were successfully synthesized using a method in a previous report.28 The microstructure of HMSN was characterized using TEM, and it was found that they were composed of a uniform, huge hollow cavity in the core and there was a well defined nanoporous silica shell (Fig. 1a). The particle size distribution of the HMSN was found to be relatively narrow. The average diameter was about 265 ± 13 nm and the average thickness of the mesoporous shell was determined to be about 43 ± 7 nm. It was also observed that the mesopores were homogeneously arranged, which gave efficient channels for loading drugs. The narrow size distribution of the nanoparticle was also confirmed using dynamic light scattering (DLS) measurements (Fig. S1; ESI†).To investigate the availability of the pore structure from the synthesized HMSN, the nitrogen (N2) adsorption/desorption isotherms were measured (Fig. S2a; ESI†). The isotherms of the samples exhibited characteristic type IV, N2 adsorption/desorption patterns, indicating the presence of mesopore channels in HMSN. The specific surface area and pore volume were calculated to be 537.1 m2 g−1 and 0.6012 cm3 g−1, respectively, and the average pore size was about 4.48 nm (Fig. S2b; ESI†).
3.2 Loading and drug release assay in vitro
To obtain DMSN, DOX was loaded into the HMSN via the electrostatic interaction between the negatively charged silica surface and the positively charged DOX molecules.29 The loading efficiency of DOX in DMSN was 9.4%. However, the loading efficiency of DOX in BGNS-SBC and BGNS-AC was 11.5% and 11.2%, respectively, which was higher than that of DMSN and this was attributed to the fact that the loaded DOX of DMSN can react with SBC or AC and produce a precipitate. The morphology of the BGNS-SBC or BGNS-AC were characterized using TEM (Fig. 1b and c). The homogeneous size distribution of the BGNS-AC and BGNS-SBC were also revealed using DLS measurements (Fig. S1, Table S1; ESI†). By comparison with the hollow mesopores of HMSN, the mesopores of BGNS-SBC and BGNS-AC were filled and this indicated the successful loading of DOX. The zeta potentials of HMSN, DMSN, BGNS-SBC and BGNS-AC were −25.4 ± 4.84 mV, 28.7 ± 5.04 mV, −8.67 ± 3.74 mV and −22.1 ± 3.75 mV (Table S1; ESI†), respectively. This difference in zeta potential between DMSN, BGNS-SBC and BGNS-AC is attributed to the formation of bicarbonate and carbonate deposition.The in vitro release of DOX was examined in PBS with different pH values. Fig. 2 shows that BGNS-SBC and BGNS-AC could release DOX in a pH responsive release manner. In PBS at pH 7.4, the cumulative release of DOX was only about 11.3% for BGNS-SBC, and 6.2% for BGNS-AC within 48 h. This result showed that there was a small amount of drug leakage in normal physiological conditions. However, in PBS at pH 5.0, which was used to simulate the intracellular lysosomal conditions of the cells, the release rate of DOX became much faster. The cumulative release of DOX from BGNS-SBC and BGNS-AC could reach as high as about 51.4% and 44.9%, respectively, within 48 h. This result suggested that upon internalization by cells, BGNS-SBC can release DOX faster than BGNS-AC in acidic intracellular compartments. The amount of DOX release increased together with the increasing incubation time in an acidic environment. TEM showed a corresponding result with the drug release profile. Treating both BGNS-SBC and BGNS-AC with PBS at pH 7.4 for 48 h led to the observation of almost no hollow structure (Fig. S3; ESI†). However, after incubating the drug delivery systems with PBS at pH 5.0 for 48 h, more particles with a hollow structure could be observed. This result indicated that BGNS-SBC and BGNS-AC were stable at normal physiological conditions but can effectively release DOX in acidic environments.
3.3 In vitro cytotoxicity studies
A high biocompatibility of the drug delivery system was required for its application in cancer treatment. MCF-7 cells and MCF-7/ADR cells were incubated with HMSN for 24 h or 48 h and then the cell viability was investigated using the CCK-8 assay. As shown in Fig. S4 (ESI†), no apparent cytotoxicity was observed. The viability of the cells was still above 80% even after incubation with a high concentration of HMSN (200 μg mL−1).Subsequently, the cytotoxic activity of free DOX and BGNS was determined. MCF-7 cells were incubated with a series of equivalent concentrations (0.8 to 25.6 μg mL−1) of free DOX, BGNS-SBC and BGNS-AC for 24 and 48 h (Fig. 3a and b). After treatment for 24 h, the 50% inhibiting concentration (IC50) values of free DOX, BGNS-SBC and BGNS-AC were 3.8 ± 0.35 μg mL−1, 7.6 ± 0.26 μg mL−1 and 11.3 ± 0.61 μg mL−1, respectively. After treatments for 48 h, the IC50 values of free DOX, BGNS-SBC and BGNS-AC were 2.8 ± 0.15 μg mL−1, 5.9 ± 0.52 μg mL−1 and 8.4 ± 0.40 μg mL−1, respectively (Fig. 3b). However, BGNS-SBC was found to exhibit a higher cytotoxicity when compared to that of BGNS-AC.
To determine whether two BGNS could reverse MDR, the cytotoxicity of DOX, BGNS-SBC and BGNS-AC were evaluated against MCF-7/ADR cells. As shown in Fig. 3c and d, free DOX did not exhibit visible cytotoxicity at a low concentration (0 to 12.6 μg mL−1), the MCF-7/ADR cells viability was still above 60% after 24 and 48 h, with treatment with DOX at a high concentration (25.6 μg mL−1). This phenomenon could be attributed to the overexpression of P-gp on the cell membrane of MCF-7/ADR which pumped the drug out.30 Compared with free DOX, BGNS showed remarkable cytotoxicity after 48 h incubation with the cells. The IC50 values of free DOX, BGNS-SBC and BGNS-AC were 25.7 ± 1.51 μg mL−1, 11.4 ± 1.33 μg mL−1 and 15.1 ± 1.65 μg mL−1. Similar results were observed for 24 h treatment, and the IC50 values of free DOX, BGNS-SBC and BGNS-AC were 36.3 ± 1.72 μg mL−1, 19.4 ± 1.26 μg mL−1 and 23.5 ± 1.13 μg mL−1, respectively. The therapeutic efficacy of BGNS-SBC and BGNS-AC was higher than that of free DOX. In addition, the BGNS-SBC was found to be more cytotoxic than BGNS-AC at the same DOX concentrations. These cytotoxicity assays indicate that BGNS-SBC and BGNS-AC have an excellent anti-drug resistance potential.
3.4 Ultrasound images assay
The pH responsive characteristics of the drug delivery system were visualized by examining the generation of CO2 bubbles in a steel pipe containing BGNS-SBC or BGNS-AC in PBS with different pH values (7.4 and 5.0) at 37 °C.31 As shown in Fig. 4, no CO2 bubbles were generated from BGNS-SBC or BGNS-AC in a pH 7.4 solution. In contrast, a large number of bubbles formed when BGNS-SBC and BGNS-AC were treated with a pH 5.0 buffer. These observations confirm that a significantly greater number of CO2 bubbles were generated in an environment with a weak acid. However, BGNS-SBC generated more bubbles at a higher speed than BGNS-AC. This is because bicarbonate reacts faster with protons than carbonate. | ||
| Fig. 4 Ultrasound images of BGNS-AC (I) and BGNS-SBC (II) after incubation in pH 5.0 and pH 7.4 PBS solutions. | ||
3.5 Confocal laser-scanning microscopy (CLSM) studies
CLSM was applied to visualize the cellular uptake and intracellular DOX release in MCF-7 cancer cells. The red fluorescence indicated the distribution of DOX, the green fluorescence indicated the location of lysosomes, which were stained using LysoTracker® Green, and the blue stain indicated the nuclei stained using Hoechst 33342. After incubating the cells with free DOX for 4 h (Fig. S5a; ESI†), red fluorescence can be observed in the nuclei, which shows the fast internalization of DOX. The distribution of DOX did not change as time went on (Fig. S5; ESI†). In comparison, as shown in Fig. 5, after 12 h incubation, red fluorescence of DOX from BGNS-SBC or BGNS-AC was mostly localized in the lysosomes. Similar results were observed after 24 h incubation (Fig. S5b; ESI†). These experiments demonstrate that the diffusion of free DOX into cell nuclei is a relatively fast process, whereas the DOX of BGNS-SBC or BGNS-AC mainly accumulated in the lysosomes. These results indicated that the DOX of both types of BGNS had almost no effect on killing cancer cells for its effect is to interact with DNA.32 | ||
| Fig. 5 Cellular co-localization images of intracellular DOX distribution in MCF-7 cells after incubation with DOX (I), BGNS-AC (II) and BGNS-SBC (III) for 12 h. | ||
3.6 LMP studies
Enhanced LMP may cause the release of certain cathepsins into the cytoplasm from the lysosome and then this triggers cell death by apoptosis or an apoptosis-like path.14 To investigate the effect of DOX, BGNS-SBC and BGNS-AC on the lysosomes, changes in LMP were visualized using a fluorescence imaging method described in a previously reported method.33 With increasing LMP, the fluorescent dextran progressed from a punctate lysosomal pattern to diffused cytosolic staining. As shown in Fig. 6, free DOX increased the LMP slightly, whereas BGNS-SBC and BGNS-AC dramatically enhanced LMP. This can be attributed to the fact that BGNS-SBC and BGNS-AC generated a great number of CO2 bubbles inside the lysosomes of MCF-7 cells. The generated bubbles produced sufficient force to expand the lysosome, and as a result, enhanced LMP occurs. Bicarbonate reacts with acids more quickly than carbonate, thus, BGNS-SBC increased LMP more effectively than BGNS-AC. | ||
| Fig. 6 Visualisation of lysosome membrane permeabilization [**p < 0.01, and ***p < 0.001, when compared with drug treated cells and with untreated cells (control)]. | ||
3.7 In vitro assessment of caspase-3 activity
Caspase-3 is a key enzyme in the process of apoptosis and the up-regulation of active caspase-3 is evidence of apoptosis.3 In this research, the caspase-3 activity was also investigated after incubating the MCF-7 cells with free DOX, BGNS-SBC and BGNS-AC. Incubation with free DOX induced a slight increase of caspase-3 activity compared with the control group, whereas BGNS-AC caused a higher increase and BGNS-SBC caused the highest increase of enzyme activity (Fig. 7). The higher the value of caspase-3, the more apoptosis of the cancer cells occurs. These observations confirm that apoptosis may be the mechanism underlying tumor cell death for BGNS.4 Conclusions
In summary, two types of H+-triggered bubble generating drug delivery systems, i.e., BGNS-SBC and BGNS-AC, were designed and fabricated. It was found that BGNS-SBC and BGNS-AC could enhance LMP and induce the up-regulation of caspase-3 activity by releasing CO2 bubbles in the intracellular lysosome which then resulted in cell death. In addition, when compared to free drug molecules, BGNS-SBC and BGNS-AC displayed a powerful inhibition against drug resistant cells and enhanced antitumor efficacy. More interesting is the fact that the cytotoxicity of BGNS-SBC was higher than that of BGNS-AC against drug resistant cells. These results demonstrated that there is a great potential for overcoming cancer drug resistance and further research will also help advance the clinical relevance of these findings.Acknowledgements
This work was supported by the National Key Scientific Program – Nanoscience and Nanotechnology (No. 2011CB933600), the National Natural Science Foundation of China (No. 21175110, 21405124), and the Fundamental Research Funds for the Central Universities (No. XDJK2013A022, XDJK2014C173, XDJK2016E057). The authors thank Prof. Xie Haiyan and Dr Zhang Fan (Beijing Institute of Technology) for their assistance with the cellular co-localization imaging experiment.Notes and references
- S. P. Cole, G. Bhardwaj, J. H. Gerlach, J. E. Mackie, C. E. Grant, K. C. Almquist, A. J. Stewart, E. U. Kurz, A. M. Duncan and R. G. Deeley, Science, 1992, 258, 1650–1654 CrossRef CAS PubMed.
- D. Longley and P. Johnston, Biochem. J., 1990, 272, 281–295 CrossRef.
- S. E. Bates, Nat. Rev. Cancer, 2002, 2, 48–58 CrossRef PubMed.
- H. M. Coley, Cancer Treat. Rev., 2008, 34, 378–390 CrossRef CAS PubMed.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- L. Pan, Q. He, J. Liu, Y. Chen, M. Ma, L. Zhang and J. Shi, J. Am. Chem. Soc., 2012, 134, 5722–5725 CrossRef CAS PubMed.
- H. Wu, W. N. Hait and J.-M. Yang, Cancer Res., 2003, 63, 1515–1519 CAS.
- N. J. Roberts, L. Zhang, F. Janku, A. Collins, R.-Y. Bai, V. Staedtke, A. W. Rusk, D. Tung, M. Miller and J. Roix, Sci. Transl. Med., 2014, 6, 249ra111 CrossRef PubMed.
- Y. Lin, H. Zhang, J. Liang, K. Li, W. Zhu, L. Fu, F. Wang, X. Zheng, H. Shi and S. Wu, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E4504–E4512 CrossRef CAS PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- R. D. Hofheinz, S. U. Gnad-Vogt, U. Beyer and A. Hochhaus, Anti-Cancer Drugs, 2005, 16, 691–707 CrossRef CAS PubMed.
- C. D. Duve, Eur. J. Biochem., 1984, 137, 391–397 CrossRef.
- U. Repnik, M. H. Česen and B. Turk, Mitochondrion, 2014, 19, 49–57 CrossRef CAS PubMed.
- P. Boya and G. Kroemer, Oncogene, 2008, 27, 6434–6451 CrossRef CAS PubMed.
- P. Saftig and K. Sandhoff, Nature, 2013, 502, 312–313 CrossRef CAS PubMed.
- M. T. Gyparaki and A. G. Papavassiliou, Trends Mol. Med., 2014, 20, 239–241 CrossRef CAS PubMed.
- S. P. Hudson, R. F. Padera, R. Langer and D. S. Kohane, Biomaterials, 2008, 29, 4045–4055 CrossRef CAS PubMed.
- X. Ma, Q. Qu and Y. Zhao, ACS Appl. Mater. Interfaces, 2015, 7, 10671–10676 Search PubMed.
- A. Z. Wang, R. Langer and O. C. Farokhzad, Annu. Rev. Med., 2012, 63, 185–198 CrossRef CAS PubMed.
- C. Yu, M. Zhou, X. Zhang, W. Wei, X. Chen and X. Zhang, Nanoscale, 2015, 7, 5683–5690 RSC.
- S. Santra, C. Kaittanis, O. J. Santiesteban and J. M. Perez, J. Am. Chem. Soc., 2011, 133, 16680–16688 CrossRef CAS PubMed.
- J. Ghosh, J. Das, P. Manna and P. C. Sil, Biomaterials, 2011, 32, 4857–4866 CrossRef CAS PubMed.
- J. Z. Du, X. J. Du, C. Q. Mao and J. Wang, J. Am. Chem. Soc., 2011, 133, 17560–17563 CrossRef CAS PubMed.
- B. Y. Choi, H. J. Park, S. J. Hwang and J. B. Park, Int. J. Pharm., 2002, 239, 81–91 CrossRef CAS PubMed.
- C. J. Ke, T. Y. Su, H. L. Chen, H. L. Liu, W. L. Chiang, P. C. Chu, Y. Xia and H. W. Sung, Angew. Chem., Int. Ed., 2011, 50, 8086–8089 CrossRef CAS PubMed.
- C. J. Ke, W. L. Chiang, Z. X. Liao, H. L. Chen, P. S. Lai, J. S. Sun and H. W. Sung, Biomaterials, 2013, 34, 1–10 CrossRef CAS PubMed.
- L. Yang, Z. Wen, Y. Long, N. Huang, Y. Cheng and H.-Z. Zheng, Chem. Commun., 2016, 52, 10838–10841 RSC.
- Y. Gao, Y. Chen, X. Ji, X. He, Q. Yin, Z. Zhang, J. Shi and Y. Li, ACS Nano, 2011, 5, 9788–9798 CrossRef CAS PubMed.
- S. N. Shanta, H. Kulkarni, L. Pradhan and D. Bahadur, Nanotechnology, 2013, 24, 193–198 Search PubMed.
- Y. Gao, L. Chen, Z. Zhang, Y. Chen and Y. Li, Biomaterials, 2011, 32, 1738–1747 CrossRef CAS PubMed.
- C. Hsin-Cheng, Y. W. Lin, Y. F. Huang, C. Chih-Kai and C. Chorng-Shyan, Angew. Chem., Int. Ed., 2007, 47, 1875–1878 Search PubMed.
- S. Cai, S. Thati, T. R. Bagby, H.-M. Diab, N. M. Davies, M. S. Cohen and M. L. Forrest, J. Controlled Release, 2010, 146, 212–218 CrossRef CAS PubMed.
- N. T. Petersen, O. Olsen, L. Groth-Pedersen, A. M. Ellegaard, M. Bilgin, S. Redmer, M. Ostenfeld, D. Ulanet, T. Dovmark and A. Lønborg, Cancer Cell, 2013, 24, 379–393 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1; Fig. S1–S4. See DOI: 10.1039/c6ra19863e |
| This journal is © The Royal Society of Chemistry 2016 |