Preparation of ellipsoid-shaped supraparticles with modular compositions and investigation of shape-dependent cell-uptake†
S. Ulrich a,
C. Hirschb,
L. Dienerb,
P. Wickb,
R. M. Rossia,
M. B. Bannwarth*a and
L. F. Boesel*a
a,
C. Hirschb,
L. Dienerb,
P. Wickb,
R. M. Rossia,
M. B. Bannwarth*a and
L. F. Boesel*a
aEmpa, Swiss Federal Laboratories for Materials Science and Technology. Laboratory for Protection and Physiology. Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland. E-mail: Luciano.Boesel@empa.ch; bannwart@mpip-mainz.mpg.de
bEmpa, Swiss Federal Laboratories for Materials Science and Technology. Particle-Biology Interactions Laboratory. Lerchenfeldstrasse 5, CH-9014 St. Gallen, Switzerland
First published on 12th September 2016
Abstract
Hybrid colloidal supraparticles often show a superior performance in catalysis, optics and biomedicine thanks to the synergistic effect of the ensemble of their single nanoparticle building blocks. Despite the emerging importance of shape-dependent properties of nanostructures, the synthesis of supraparticles is generally limited to a spherical shape. Here, a broadly applicable method is presented for the fabrication of ellipsoid supraparticles from one or several types of inorganic nanoparticles in various compositions. The method is highly versatile and modular, allowing free choice of hydrophobic nanoparticles to combine desired properties in the resulting supraparticles. A representative series of ellipsoid-shaped supraparticles is fabricated and their morphology, hybrid structure and composition as well as their functional properties are investigated. All employed nanoparticle types are successfully incorporated resulting in ellipsoid-shaped supraparticles with largely homogeneous intra- and interparticular distribution of the different nanoparticle building blocks. A biological assessment of iron oxide ellipsoid supraparticles reveals no safety issues but a pronounced lower cellular uptake compared to spherical ones. This distinct shape–property relationship illustrates the importance of the supraparticle shape as a parameter for the rational design of nanosystems for biomedical applications.
1. Introduction
The assembly of nanoparticles into colloidal nanoclusters, so-called supraparticles (SP), has been the subject of strong scientific interest in recent years.1–3 These SPs can concentrate the functional properties of the single nanoparticles in their cluster structure thereby enhancing their collective effect, or they may even possess new properties due to synergistic effects stemming from the close proximity of their nanoparticle building blocks.4 Of special interest is the combination of different nanoparticle types with selected properties into hybrid SPs to unite different properties in one nanostructure. Depending on the properties of the nanoparticle building blocks, possible applications of SPs range from the biomedical field5–7 to optics8 and heterogeneous catalysis.9 For example, fluorescence and superparamagnetism combined in one SP could be employed for multi-modal imaging.5,10 Hence, in the last decade, a variety of methods for the fabrication of SPs have been established.3In recent years, a distinct relationship between shape and function of nanoparticles has been discovered in several areas. In the biomedical field, the shape of nanoparticles in addition to size and surface chemistry has emerged as an important parameter that influences their behavior in living organisms as well as their uptake into different types of cells.11–13 Consequently, their applicability in the field of nanomedicine is also strongly dependent on their shape and the unclear shapes-to-property relationship requires further investigations. Magnetic nanomaterials with non-spherical shapes, in particular, are of high interest since they may possess anisotropic, shape-dependent magnetic properties such as the ability to orient themselves in a magnetic field.14 Furthermore, they have been shown to perform better in magnetic hyperthermia15 and have been used as magnetic photonic ink16 and, recently, Yang et al.17 demonstrated that magnetic nanochains functionalized with palladium nanoparticles can simultaneously act as heterogeneous catalysts and magnetic nanostirbars.
Hence, multifunctional anisotropic SPs are highly desirable for potential applications in a variety of fields. Over the last two decades, a broad variety of methods have been reported for the shape-controlled synthesis of single nanoparticles.18 For example, through controlled crystal growth, a large abundance of nanoparticle with various shapes becomes available.19,20 For SPs, however, a shape control combined with a free choice of nanoparticle composition, to the best of our knowledge, has not been reported to date. Hybrid one-dimensional nanomaterials rely either on the laborious post-synthetic functionalization of single crystal nanoellipsoids,21,22 on magnetic nanochains assembled from spherical magnetic nanoclusters,23,24 or on stretching of polymer-supported inorganic nanoparticles in a PVA matrix.25 All of these methods possess different drawbacks regarding restrictions on the available properties or the possible building blocks. Ideally, the methods for the versatile and modular assembly of multi-functional SPs would also allow the generation of non-spherical shapes. However, due to energetic factors governing the assembly processes, these methods have been almost exclusively restricted to spherical SPs.3 Very few non-spherical examples exist that are based on the specific and complex assembly of anisotropically-shaped nanoparticles in cylindrical26 or cubic27 supercrystalline SPs.
We have recently shown how the uniaxial stresses that occur during emulsion electrospinning can be employed to fabricate ellipsoid-shaped SPs from magnetic nanoparticles by an elongation–evaporation process.28 In this paper, we present a newly developed method for the modular and rapid fabrication of ellipsoid supraparticles (ESPs) consisting of only one nanoparticle type and of hybrid ellipsoid supraparticles (HESPs) composed of several different nanoparticle types. To our knowledge, this is the first report of a broadly applicable method to generate ellipsoid supraparticles from various inorganic nanoparticles or combinations thereof. The method is highly modular, allowing a free choice of hydrophobic nanoparticle combinations depending on the required properties of the resulting HESPs. Furthermore, to emphasize the importance of shape for function, we employ ESPs from superparamagnetic nanoparticles, a material of high interest for a range of biomedical applications,29 as a model system to address, for the first time, the influence of the shape of supraparticles on cellular uptake. While both particle types do not induce cytotoxic effects under the experimental conditions, our results show that, indeed, ellipsoids SPs deviate from their spherical counterparts as they showed lower cellular uptake by the lung epithelial carcinoma cell line A549.
2. Experimental
2.1 Materials
Hydrophobic titanium dioxide nanoparticles (TiO2NP) and hydrophobic silver nanoparticles (AgNP) were purchased from Plasmachem. Commercial silica nanoparticles SiO2NP were hydrophobized according to a protocol reported elsewhere.30 SDS, n-octane, PVA Mowiol 40–88 (Mw = 205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), iron(III)chloride hexahydrate, iron(II)chloride tetrahydrate, trypsin–EDTA, RPMI-1640 medium, phosphate buffered saline (PBS), bovine serum albumin (BSA), and ammonia solution were purchased from Sigma Aldrich. Fetal bovine serum (FCS), L-glutamine, penicillin, neomycin, and streptomycin were purchased from Gibco. Oleic acid was purchased from Fluka Analytical. Chloroform and toluene were purchased from Fischer Scientific. All solvents were of analytical grade and used as received. Deionized water was used in all experiments and measurements (resistivity 18.2 MΩ cm).
000 g mol−1), iron(III)chloride hexahydrate, iron(II)chloride tetrahydrate, trypsin–EDTA, RPMI-1640 medium, phosphate buffered saline (PBS), bovine serum albumin (BSA), and ammonia solution were purchased from Sigma Aldrich. Fetal bovine serum (FCS), L-glutamine, penicillin, neomycin, and streptomycin were purchased from Gibco. Oleic acid was purchased from Fluka Analytical. Chloroform and toluene were purchased from Fischer Scientific. All solvents were of analytical grade and used as received. Deionized water was used in all experiments and measurements (resistivity 18.2 MΩ cm).
2.2 Synthesis of oleate-coated iron oxide nanoparticles
Oleate-coated iron oxide nanoparticles Fe3O4NP were synthesized by a standard co-precipitation protocol.28 Iron(III) chloride hexahydrate (24.36 g, 90 mmol) and iron(II)chloride tetrahydrate (12.01 g, 60 mmol) were dissolved in water (100 mL). Oleic acid (4.00 g, 14 mmol) was added to the solution followed by an aqueous ammonia solution (40 mL, 28 wt%) which was added drop-wise under rigorous stirring. Subsequently, the solution was heated to 70 °C in an oil bath under constant stirring for one hour and two hours at 110 °C. To account for evaporating water, it was refilled constantly. After the oleate-capped iron oxide nanoparticles precipitated, they were rinsed with water several times and dried in an oven at 65 °C overnight.2.3 Preparation of nanoparticle-loaded miniemulsions
For the formation of nanoparticle-containing miniemulsion droplets, the nanoparticle types were separately dispersed in chloroform (total amount of chloroform 4.5 g) or, if possible, in n-octane (1 g) or toluene (1 g) under ultrasonication. The quantities of the nanoparticles depend on the intended composition of the SPs and were between 30 mg and 1 g. The dispersions were subsequently combined (total amount of nanoparticles 300 to 1000 mg) followed by the addition of n-octane (0.5 g) under ultrasonication. The dispersion was mixed with an aqueous solution of SDS (15 mL, 0.2 wt%) by vortexing for several seconds and sonified using a Branson Digital Sonifier with a 1/2 inch ultrasonication tip under ice cooling (3 min, 50% amplitude, 10 s pulse, 5 s pause) followed by the addition of SDS (50 mg). The miniemulsion was immersed in an oil bath at 40 °C or 80 °C for chloroform/n-octane or toluene/n-octane, respectively, stirred magnetically (600 rpm) for 1 h. For miniemulsion droplets containing Fe3O4NP, the droplets were subsequently magnetically separated and redispersed in aqueous SDS solution (0.3 wt%).2.4 Emulsion electrospinning
The emulsion electrospinning process and setup were described before.28 The obtained miniemulsion was combined with a PVA stock solution (15 wt%) for a total PVA concentration of 10 wt% and mixed mechanically. The resulting emulsion was filled into a 1 mL plastic syringe and electrospun in a climate chamber at 60% relative humidity and 24 °C onto an aluminum foil support (20 cm distance, 5 μL min−1 feed rate, 0.8 mm tip diameter, +12 kV and −5 kV and 0.1 mA). A custom made electrospinning setup was used. In brief, an infusion pump (KD Scientific, USA) provided a constant emulsion flow through a plastic syringe (1 mL, Henke Sass Wolf, Germany) equipped with a stainless steel needle (0.8 mm inner diameter, Unimed S.A., Switzerland). Positive and negative voltage supply sources (AIP Wild AG, Switzerland), were connected to the needle and the collector respectively. The device was placed in a Faraday cage, which was integrated in a fume hood in a climatic chamber.2.5 PVA fiber dissolution
To release the ESPs and HESPs from the PVA fibers to an aqueous dispersion, the fiber mesh was removed from the aluminum support and the PVA was dissolved in aqueous SDS solution (0.3 wt%) over 2 h. Subsequently, magnetic SPs were magnetically separated on a strong permanent magnet, or, for non-magnetic SPs, centrifuged (20 min at 2000 rcf) followed by redispersion in aqueous SDS solution. They were stored at room temperature for up to four months without observable changes in structure (STEM). If the HESPs were partially sedimented after long storage, they could be easily redispersed by vortexing for few seconds.2.6 Calcination of HESPs
Calcination of HESPs was performed by drop-casting the dispersion on a silicon wafer and heating in an oven. The temperature was ramped up over 1 h to 600 °C and kept at that temperature for 6 h.2.7 Magnetic orientation of ESPs and HESPs
For the magnetic orientation of ESPs and HESPs, dispersions diluted with water were drop-casted on STEM grids and placed at 4 cm (ESP) or 2 cm (HESP) away from a strong NdFeB permanent magnet (40 × 40 × 20 mm). The water was left to evaporate at room temperature.2.8 Preparation of ESPs of Fe3O4 and spherical nanoclusters for cellular uptake analysis
A miniemulsion was prepared from Fe3O4NP as described above. It was diluted with SDS (0.1 wt%) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and centrifuged (239 rcf) for 25 min. The supernatant was used for the fabrication of ESPs of Fe3O4 as described above. For a spherical nanocluster reference, the same supernatant (70 μL) were mixed with SDS solution (4 mL, 0.3 wt%) and heated in an oil bath at 80 °C for one hour. Both ESPs and spherical nanoclusters were transferred to water by magnetic sedimentation and redispersion in water. Iron concentrations of the dispersions were determined by ICP-OES (see below).
1 and centrifuged (239 rcf) for 25 min. The supernatant was used for the fabrication of ESPs of Fe3O4 as described above. For a spherical nanocluster reference, the same supernatant (70 μL) were mixed with SDS solution (4 mL, 0.3 wt%) and heated in an oil bath at 80 °C for one hour. Both ESPs and spherical nanoclusters were transferred to water by magnetic sedimentation and redispersion in water. Iron concentrations of the dispersions were determined by ICP-OES (see below).
Cell culture: the human alveolar epithelial cell line A549 (ATCC: CCL-185) was grown in complete cell culture medium consisting of RPMI-1640 medium supplemented with 10% (v/v) FCS, 0.2 mg mL−1 L-glutamine, 50 μg mL−1 penicillin, 50 μg mL−1 streptomycin, and 100 μg mL−1 neomycin under standard growth conditions (humidified air, 37 °C, 5% CO2). Cells were subcultured routinely twice a week at approximately 80–90% confluency using 0.5% trypsin–EDTA.
2.9 Transmission electron microscopy and ICP-OES of cells
Per well of a 6 well cell culture plate 4 × 105 A549 cells were seeded in complete cell culture medium and grown overnight under standard growth conditions. Cells were treated with 2.5 mL complete cell culture medium containing 10 μg mL−1 SPs per well for 24 hours, detached with 0.5% trypsin–EDTA and pelleted by centrifugation (200g, 5 min). For TEM analysis pelleted cells were sucked up into a capillary tube (Leica-Microsystems, Heerbrugg, Switzerland). Therein cells were fixed in 3% glutaraldehyde in a 0.1 M sodium cacodylate buffer. After a post-fixation step in 2% osmium tetroxide in 0.1 M sodium cacodylate buffer, cells were dehydrated through a graded ethanol series followed by acetone and finally embedded in Epon resin (Fluka). Ultrathin sections were contrasted with 2% uranyl acetate and lead citrate before observation in a Zeiss EM 900 (Carl Zeiss Microscopy, GmbH, Germany) at 80 kV.31 For ICP-OES measurements cell pellets were resuspended in complete cell culture medium and counted using a Neubauer counting chamber. Equal amounts of cells for each sample were pelleted again (200g, 5 min) and processed for ICP-OES measurements (more detailed description in the ESI†).2.10 Flow cytometry
A549 cells were grown for 24 h in 12-well cell culture plates at a density of 2 × 105 cells per well under standard growth conditions. Cells were treated for 24 h in 1 mL of complete cell culture medium containing 1, 3 or 10 μg mL−1 SPs. Thereafter, cells were detached with 0.5% trypsin–EDTA, pelleted by centrifugation (200g, 5 min) and the resulting pellet was resuspended in 300 μL PBS containing 0.5 wt% BSA. 100 μL of this cell suspension were used in 1 mL of PBS for flow cytometric analysis using a Gallios flow cytometer (Beckman Coulter) and Kaluza software. 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per sample were analyzed. Forward scatter was plotted linearly while side scatter values were plotted logarithmically.
000 cells per sample were analyzed. Forward scatter was plotted linearly while side scatter values were plotted logarithmically.
2.11 Instrumental analysis
SEM, EDX, and STEM were performed on a Hitachi S-4800 (Hitachi High technologies, Canada). For SEM, dispersions were drop-casted on silicon wafers. Electrospun membranes were directly attached to the sample support. SEM samples were sputtered with 5 nm gold/palladium. For EDX measurements of bulk compositions of HESPs, the dispersions were drop-casted on silicon wafers or on a piece of plastic foil (Parafilm) and coated with 10 nm carbon. Sputtering and carbon coating were performed on a SEM coating Unit (Polaron Equipment, E5100, Kontron AG, Switzerland). For EDX point measurements in single HESPs, dispersions were diluted in water and drop-casted on 300 mesh carbon coated copper grids (Plano GmbH). For STEM samples, dispersions were diluted in water and drop-casted on a 300 mesh carbon coated copper grid or, for nanofibers, electrospun directly on the copper grids. XRD patterns were obtained using a PANalytical X'Pert PRO θ–2θ scan system equipped with a Johansson monochromator (Cu-Kα1 radiation, 1.5406 Å) and an X'Celerator linear detector. The diffraction pattern was recorded between 20° and 80° (2θ) with an angular step interval of 0.0167°. TGA was performed on a NETZSCH TG 209 F1 instrument under nitrogen atmosphere from 25 to 900 °C with a heating rate of 10 K min−1. ICP-OES determination of the iron content in dispersions were performed on a Optimus 3000 (Perkin-Elmer). Nanocluster dispersions (50 μL) were mixed with 100 μL concentrated nitric acid (69%) for 30 min before the sample was diluted for the measurement. Flow cytometry measurements to determine inorganic content in cells were performed on a Gallios flow cytometer (Beckman Coulter) with Kaluza software. Magnetic properties were determined via a SQUID magnetometer (Quantum Design MPMS XL). The sample material was measured in a gelatin capsule, which provides a small mainly diamagnetic background signal. An empty capsule was measured separately at identical conditions and its contribution to the measured signal is subtracted in the shown data.3. Results and discussion
3.1 Detailed work procedure and process optimization
We have previously demonstrated how prolate ESPs can be fabricated by an elongation–evaporation process during emulsion electrospinning. For this purpose, we electrospun miniemulsions containing droplets that were highly loaded with superparamagnetic nanoparticles.28 Based on our previous results, we hypothesized that this approach could be employed to create ESPs from different inorganic nanoparticle types or even hybrid ellipsoid supraparticles HESPs unifying several nanoparticle types with various properties in one SP. However, the necessity of the high-boiling and ultrahydrophobic n-octane for the original process severely limits its applicability towards other inorganic nanomaterials. Owing to the low degree of redispersion of most inorganic nanoparticles in n-octane, they can't be inserted into the process. Most hydrophobic nanoparticles disperse very well in chloroform and also toluene, making them much more versatile solvents for our purpose. To enable the insertion of a broad range of nanoparticles in the electrospinning-induced elongation–evaporation process, we combined solvent mixtures with a partial evaporation step and were able to develop a broadly applicable method for the creation of ESPs and HESPs.The detailed work procedure is described in Fig. 1. Four kinds of hydrophobic nanoparticles (Fig. 1A and ESI Fig. S1†) were used for this work alone and in varying combinations. We selected superparamagnetic iron oxide nanoparticles (Fe3O4NP), anatase phase titanium dioxide nanoparticles (TiO2NP), silica nanoparticles (SiO2NP), and metallic silver nanoparticles (AgNP) due to their distinct properties. Silver and anatase nanoparticles possess optical properties with well-defined light absorption (ESI Fig. S2 and S3†). Anatase is known for its photocatalytic activity which is a desired property for the incorporation into SPs.9 Fe3O4NP are superparamagnetic and SPs thereof possess enhanced properties in biomedical applications including magnetic separation, Magnetic Resonance Imaging (MRI) and magnetic hyperthermia.29,32 Silica nanoparticles have a long history of being functionalized with a variety of substances, fluorescent organic dyes being a common example.33,34 Additionally, bare silica nanoparticles are often applied to adjust mechanical properties in polymer hybrid systems.35 Fig. 1B schematically depicts the fabrication process. All nanoparticles are dispersed separately in chloroform in high solid contents before combining them in the desired ratios. Then, about 10 wt% n-octane relative to chloroform is added. Alternatively, toluene can be used instead or in addition to chloroform depending on the nanoparticle type or, if all are dispersible in n-octane, they can be dispersed only therein. After combining the dispersion with a solution of sodium dodecylsulfate (SDS) in water and ultrasonication treatment, a miniemulsion is obtained. The miniemulsion is heated carefully to evaporate predominantly the chloroform which is possible due to the difference in boiling points of about 60 °C. Toluene can be predominantly removed from the droplets due to the formation of an azeotrope with water that allows it to diffuse out upon heating. Removing the chloroform from the droplets avoids the formation of chloroform bubbles in the syringe during the electrospinning but, additionally, the increased density of the emulsion droplets also allows for magnetic concentration and purification of the miniemulsion when containing Fe3O4NPs. After mixing with a poly(vinyl alcohol) (PVA) solution, the miniemulsion is inserted into the electrospinning process. During electrospinning, the droplets are incorporated into the liquid jet from which fibers are formed. Formation of the electrospun fibers through thinning of the liquid jet and water evaporation is accompanied by strong longitudinal stresses along the jet axis which have been estimated to be in the range of 10–100 kPa.36 These forces cause a deformation of the miniemulsion droplets into an ellipsoid shape. Just like the evaporation of water results in the formation of a solid fiber, the evaporation of the n-octane from the deformed droplets results in a permanent elongated state with the HESPs now possessing a stable cluster structure that is completely embedded in the PVA fiber. The process highlights the importance of the properties of the organic solvent. The solvent should not evaporate too fast to allow for droplet deformation during the fiber formation. However, it must evaporate fast enough to form stable ellipsoid SPs from the deformed droplets during the spinning process. The typical morphology of these PVA nanofibers can be observed in a Scanning Electron Microscopy (SEM) image of the fibers in Fig. 1C. They are homogeneous with fiber diameters in the range of 50 nm to 200 nm. With PVA being a highly water soluble polymer, the fibrous mesh containing the HESPs can subsequently be dissolved to yield the HESPs in an aqueous dispersion. Purification of the HESPs is conveniently possible by repeated magnetic separation with a strong magnet and redispersion in SDS aqueous solution or, if no Fe3O4NPs are incorporated for magnetic properties, by centrifugation and redispersion. Importantly, the whole described process from dispersing the nanoparticles to gaining the aqueous dispersions of the ESPs or HESPs is rapid and convenient, taking about four hours in total.
To identify the relevant parameters governing the process of ESP or HESP formation, we systematically investigated a variety of parameters of both the emulsion and the electrospinning process. We employed ESPs of Fe3O4 as the model system due to the convenience of magnetic purification and separation. The investigated parameters are presented in Fig. 2. The parameters can be classified by their influence on (a) the homogeneity of the fiber diameter Ø and (b) on the aspect ratio of the formed ellipsoids. We identified three main parameters that have a strong influence on the fiber homogeneity and the aspect ratio of the ESPs. The first crucial parameter is the polymer concentration of the electrospinning solution. If the polymer concentration is too low, the viscosity drops strongly in the electrospinning solution and the fibers become inhomogeneous due to Rayleigh instabilities.37,38 Hence, at lower PVA concentrations, the fibers display a beaded structure that can be observed in a Scanning Transmission Electron Microscopy (STEM) image (Fig. 2A and ESI Fig. S4†). Whereas formation of ESPs can be observed in the thinner parts of the beaded fibers, the beads themselves contain high amounts of non-elongated spherical SPs thereby strongly decreasing the overall yield of ESPs. Bead formation decreased with increasing PVA concentrations starting from 7 wt% with homogeneous fibers achieved at an optimal concentration of 10 wt% PVA. Higher concentrated solutions do not lead to further improvement but become increasingly viscous and less applicable for the electrospinning process. As a second parameter, we identified the organic solvent used for the dispersion of inorganic nanoparticles in the emulsion droplets. The solvent was found to possess the strongest influence among all parameters specifically on the formation of ESPs. We utilized n-octane, toluene, and chloroform pure and as solvent mixtures. We found a low degree of ESP formation for pure toluene (Fig. 2B) and chloroform which we attributed to an early loss of the solvent from the droplets already before fiber thinning occurs due to lower boiling points (especially chloroform) and the ability to form azeotropes with water (especially toluene). Pure n-octane and mixtures containing n-octane, however, resulted in ESP formation (images for all solvents in ESI Fig. S5†). As a third parameter, we identified the relative humidity during the electrospinning process. The relative humidity has been reported before to possess a strong influence on fiber formation from PVA and other water soluble polymers with lower values resulting in faster evaporation and thereby thicker fibers.38–41 As expected, we found generally homogeneous but slightly thicker fibers at lower relative humidity to yield a lower amount of elongated ESPs (Fig. 2C), a result which can be attributed to the strong relationship between fiber diameter and cluster elongation.28 Apart from these three main parameters, we found no observable influence of the applied voltage and the needle tip to collector distance. Variation of both parameters resulted in similar results over a wide range of values, for example, between 10 cm and 25 cm needle tip to collector distance. The amount of dispersed phase was also found to play only a minor role. The influence of the investigated process parameters is summarized in Table 1 and additional STEM images for the parameters can be found in the ESI Fig. S6–S8.† With the identification of the main parameters, we could improve the fabrication process resulting in homogeneous fibers with highly elongated ESPs, which can be observed in Fig. 2D. After dissolution of the PVA fibers in aqueous SDS solution, stable ESP dispersions were obtained that could be stored over several months at room temperature without aggregation (Fig. 2E). Furthermore, to establish better control over the size, miniemulsion droplets can be centrifuged to remove bigger droplet before the electrospinning process. Further improvement can be achieved by fractionized magnetic separation where bigger ESP reach the magnet first and, thus, can be separated. A sample of such ESP of Fe3O4 with small average sizes and high aspect ratios is shown in Fig. 3. After the initial experiments with Fe3O4NPs, we investigated the possibility to expand the process to other nanoparticles with low dispersibility in n-octane. We fabricated fiber meshes containing ESPs of TiO2 which yielded the ESPs in aqueous dispersion. Photographic images of a dispersibility test of TiO2NP in chloroform and n-octane as well as STEM images of the ESPs in PVA fibers and aqueous dispersion can be found in the ESI Fig. S9 and S10.†
| Parameter | Homogeneity/Ø of PVA fiber | Aspect ratio of ESP |
|---|---|---|
| Organic solvent | Low | Very high |
| PVA concentration | High | High |
| Relative humidity | High | High |
| Applied voltage | Low | Low |
| Distance | Low | Low |
| Dispersed phase (wt%) | Low | Low |
 | ||
| Fig. 3 STEM images of ESP of Fe3O4 with small average size and high aspect ratios which were centrifuged before electrospinning and magnetically purified to remove bigger ESP. | ||
3.2 Incorporation of different nanoparticles for hybrid ellipsoid supraparticles
We explored the possibility to unify various nanoparticles in one ellipsoid SP to form HESPs. Therefore, we exemplarily created four types of HESPs from different combinations of the four nanoparticle types to demonstrate the versatility of our method. The first two HESP types combined Fe3O4NPs with TiO2NPs or SiO2NPs, respectively, while the third type combined TiO2NPs with AgNPs. In principle, there should be no restriction for the possible combinations of nanoparticle types in one HESP system as long as the basic requirements on size and hydrophobicity are met. To demonstrate the ease with which different combinations are possible, we combined three types of nanoparticles, Fe3O4NPs, TiO2NPs, and AgNPs, at once in a fourth HESP type. Results for all experiments are shown in Fig. 4 with STEM images of all types of HESPs. For each type, the first two images show the HESPs embedded in the PVA fiber. At lower magnification (I) several fibers containing HESPs are visible whereas at high magnification in a close-up image (II), the internal structure of the single HESPs with their nanoparticle building blocks becomes observable. Finally, the last image shows the HESPs after dissolution of the PVA fiber from an aqueous dispersion (II). Due to the different contrasts of the nanoparticle types with Fe3O4NPs and AgNPs appearing much darker in STEM images than TiO2NPs and with SiO2NPs in between, the hybrid structure can be clearly observed in the STEM images. Interestingly, despite their differing solvent dispersibility, the different types of nanoparticles seem to be distributed homogeneously over the HESP structure for the most part, most clearly visible in Fig. 4D(II/III). Only in some cases, inhomogeneous areas in the HESPs are observable with higher concentrations of one type of particles (Fig. 4B(II)). For HESPs of TiO2/Ag, smaller aggregates of nanoparticles are visible in the fibers that were removed during the isolation by centrifugation after fiber dissolution. These structures were already removed for the other HESPs types by magnetic purification of the miniemulsions. Most importantly, the STEM images demonstrate that our method resulted in the formation of stable HESPs in various compositions that could be removed from the PVA fibers and transferred into aqueous dispersions without losing their structural integrity. The hydrophobic interactions between the single nanoparticles stemming from their hydrophobic ligands are strong enough to keep their structural identity even after several months in aqueous dispersion in all investigated cases.After the investigations by STEM already gave strong indications of the successful integration of different nanoparticle types into single HESPs, we used Energy Dispersive X-ray Spectroscopy (EDX) analysis to gain proof of their hybrid nature and quantitative data on their composition. Spectra from bulk EDX analysis of all HESP types are shown in Fig. 5A. Every nanoparticle type contains a signature element, iron, titanium, silicon, or silver with distinct peaks which can be clearly assigned. These peaks have been marked in Fig. 5A by colored lines indicating the respective elements. In the HESPs of Fe3O4/TiO2, the signals of titanium and iron are strong and clearly distinguishable. In the HESPs of Fe3O4/SiO2, as expected, a strong signal for silica is observable. The HESPs containing no iron oxide but titanium and silver, again, possess a strong titanium peak but, this time, no iron signal. The peak indicating silver from the AgNP can also be clearly distinguished, but is weaker as should be expected owing to the smaller fraction of AgNPs in the HESPs. A silicon artifact signal is visible as well that stems from the silicon wafer on which all HESPs which did not contain SiO2NP were measured. To avoid such an artifact signal for HESPs of Fe3O4/SiO2 they were measured on a plastic support. Finally, the last spectrum for HESPs of Fe3O4/TiO2/SiO2 that combine three different nanoparticle types of differing solvent dispersibility again shows all expected peaks, the strong signals for iron and titanium and the weaker one for the smaller fraction of silver nanoparticles. The results from EDX analysis clearly demonstrate the ability of the presented fabrication method to successfully incorporate a variety of nanomaterials into HESPs that subsequently possess almost homogeneous cluster structures as was shown above by STEM. To further investigate the distribution of nanoparticles in the single HESPs, the ratio of the elements at points along the polar axis of the HESPs was measured by EDX point measurements (Fig. 5B–E). The results show that the elements indicating the respective nanoparticles are distributed over the whole length of the HESPs. Local concentrations in the elemental distribution that could already be observed by STEM are in line with EDX results (Fig. 5B–E). However, the variability in the ratios between the point measurements are, in addition, explained by the local occurrence of single big nanoparticles at the measured spot. Overall, the results prove the successful incorporation of several nanoparticle types into single HESPs and the nanoparticle distribution over the whole structure. AgNP which were used in a low ratio compared to the other nanoparticles while often possessing relatively big sizes, as expected, show the strongest local concentrations (Fig. 5E), which was demonstrated by additional point measurements at locations of high contrast in the STEM images (Fig. 5D and E).
Furthermore, we analyzed how the ratios of nanoparticles used for the fabrication of HESPs compares to the ratio in the final HESPs. The composition of the HESPs regarding the inorganic cores of the nanoparticles can be calculated from the EDX data on the composition of the key elements, i.e. iron, titanium, silicon, and silver and the molecular formula of the oxides. However, the HESPs also consist to a relevant degree of the organic ligands that coat the single nanoparticles and separate them in the cluster structure. Hence, the ratio of inorganic to organic material for the different nanoparticle types had to be determined. We used Thermogravimetric Analysis (TGA) to determine the inorganic fraction of the nanoparticle types (thermal decomposition curves and the respective inorganic contents are shown in the ESI Fig. S11†). The combination of EDX and TGA data allowed us to calculate the composition of the HESPs. All values on the HESPs compositions, that is, the results of the elemental EDX analysis, the HESPs compositions calculated from EDX and TGA data, and the original ratio of nanoparticles for HESP fabrication are listed in Table 2. The listed results prove that all nanoparticles were successfully incorporated into the HESPs. While the ratio of nanoparticles in the HESPs differs slightly from the fabrication composition, which may be attributed to dispersibility effects, overall, the HESPs compositions resemble the composition of the original nanoparticle mixture. Furthermore, even the smallest fraction, the AgNPs, was incorporated. Accordingly, the method could be used to combine a matrix material for the main purpose of providing the stable ellipsoid SP structure with a small fraction of more valuable, highly functional nanomaterial that introduces the actual functional properties.
| HESP | Composition (relative weight) | ||
|---|---|---|---|
| Fe/Ti/Si/Aga | Nanoparticlesb | Fabricationc | |
| a Measured by EDX analysis.b Calculated from EDX and TGA data.c Ratio of nanoparticles used for fabrication. | |||
| Fe3O4/TiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.64 0.64 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.71 0.71 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Fe3O4/TiO2 calc. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.64 0.64 |
— | — |
| Fe3O4/SiO2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66 0.66 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.78 0.78 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50 0.50 |
| TiO2/Ag | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.20 0.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.14 0.14 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 0.12 |
| Fe3O4/TiO2/Ag | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.53 0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.16 0.16 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.60 0.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 0.12 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 0.12 |
As mentioned before, the HESPs possess a cluster microstructure with single nanoparticles acting as the building blocks for the ellipsoid SPs. The inorganic cores of the single nanoparticles are separated from each other by their layers of organic ligand. Hence, removal of the organic layers should result in either the collapse of the HESPs or in a nanoporous structure. The possibility to remove the organic ligands and to make the inorganic cores accessible is of importance if, for example, potential catalytic applications are to be considered. For this reason and to gain further insight into the nanoparticular structure, HESPs of type Fe3O4/TiO2 were calcinated at 600 °C for 6 h under normal atmosphere. SEM images of the HESPs before and after calcination can be observed in Fig. 6A and B at low and high magnification (see also ESI Fig. S12†). The images show how the ellipsoid shape of the HESPs is conserved during the calcination steps without loss of the structural integrity. Furthermore, at high magnification, it becomes visible how the smooth surface of the HESPs is changed upon calcination, revealing a nanoparticular structure where single nanoparticles become distinguishable. EDX data was also obtained for calcinated HESPs of Fe3O4/TiO2 (Table 2) showing that calcination does not induce compositional changes regarding the inorganic fraction, a result that is in line with the already described maintained structural integrity of the HESPs.
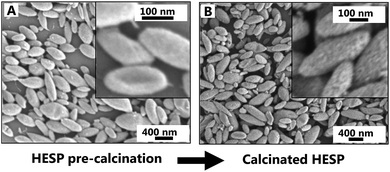 | ||
| Fig. 6 SEM images at low and high magnification of HESPs of Fe3O4/TiO2 (A) before and (B) after calcination for 6 h at 600 °C. | ||
3.3 Magnetic properties of HESPs and ESPs of Fe3O4 compared to pure superparamagnetic nanoparticles
One of the most remarkable attributes of the HESPs is the convenient combination of different nanoparticle types. Even when the focus of HESP fabrication is directed at other incorporated nanomaterials, the incorporation of magnetic nanoparticles allows for magnetic separation and thereby conveniently avoids centrifugation steps. Most importantly, however, is the combination of magnetic properties with an anisotropic ellipsoid shape which may result in anisotropic magnetic properties. Such properties add a new layer of functional behavior because magnetic fields, usually, are anisotropic by nature. We employed Superconducting Quantum Interference Device (SQUID) magnetometry to analyze the magnetic properties. We tested ESPs of only Fe3O4NP and one HESP type containing Fe3O4NPs and compared both of them to the pure Fe3O4NPs. The obtained results are shown in Fig. 7A. For all magnetization curves and, in addition, the magnetization curve for the always used Fe3O4NP in higher magnification (inset), no hysteresis is visible confirming the superparamagnetic properties of the investigated ESPs and HESPs. The magnetization curves reveal a high saturation magnetization of the original ESPs of Fe3O4NPs (50 emu g−1) for the ESPs themselves and 80 emu g−1 of the iron oxide part that is almost identical with the Fe3O4NPs on their own. The HESPs with only part of their structure consisting of Fe3O4NPs possess saturation magnetization values of 20 emu g−1 for HESPs of Fe3O4/TiO2. Overall, the results demonstrate that strong magnetic properties were introduced into HESPs by incorporating superparamagnetic nanoparticles, a result which is in line with the possibility of magnetic separation from dispersions with a strong magnet as demonstrated in Fig. 7B. The structure of the magnetic nanoparticles Fe3O4NP responsible for the magnetic properties of the HESPs was determined from X-ray Diffraction (XRD) and found to be consistent with the structure of magnetite/Fe3O4 (Fig. 7C). The most outstanding property of the HESPs besides their hybrid nature is the ellipsoid shape. To investigate how the observed magnetic properties of the ESPs and HESPs are shape-dependent, we used an evaporation-based self-assembly of magnetic ESPs and HESPs on STEM grids in the presence of a strong linear magnetic field (Fig. 7D, E and ESI Fig. S13†). Superparamagnetic nanoparticles and nanoclusters align themselves into chains, a behavior that has been long established and extensively employed for the fabrication of magnetic nanochains.23,24,42 Interestingly, our ESPs and HESPs not only align into chains but orient themselves with their long axis in the direction of the field lines much like a magnetic needle. The observed behavior demonstrates the anisotropic magnetic nature of the HESPs containing Fe3O4 that distinguish ellipsoid-shaped SPs from their spherical counterparts.3.4 In vitro biological characterization for cytotoxicity and shape-dependent cellular uptake
As discussed in the Introduction, in recent years, the shape of nanomaterials has emerged as an important parameter for their interaction with living cells. For potential applications in the biomedical field, interactions with biological systems have to be well understood to tailor a nanoparticle system to specific requirements. However, the exact effect of shape on interactions with cells and cellular uptake has not been clearly established with different studies presenting opposing results.11–13 The ESPs and HESPs present a new supraparticle system that due to its ellipsoid shape may not only be used as a model system to assess the influence of shape on cellular interactions but may additionally provide solutions for clinical needs, e.g. for multimodal imaging. We chose ESPs of Fe3O4 as a model system due to its build-up from one nanoparticle type that allows concentration determination by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES) of the iron content in dispersions. Cellular uptake is key for biomedical applications and to assess the shape-dependent interaction with cells. To start with, we chose a well-established cell line, the lung epithelial carcinoma cell line A549, which allows the comparison with the existing literature and some benchmark materials. We investigated the uptake behavior of ESPs of Fe3O4 and the respective spherical SPs. For comparability, the spherical reference sample was fabricated from the same miniemulsion by solvent evaporation but did not undergo the electrospinning process. First, to exclude acute cytotoxic effects we performed the commercially available MTS cell viability assay in A549 cells according to Rösslein et al.43 Both particle types did not influence cell viability at concentrations up to 30 μg mL−1 and over an incubation period of 24 hours (ESI†). We believe that the ellipsoid shape does not induce cytotoxic effects that where reported for other anisotropically shaped nanoparticles because the aspect ratios is lower and the diameter higher compared to nanorods that might “puncture” cell membranes.44 Transmission Electron Microscopy (Fig. 8A–C′′) revealed that after an incubation period of 24 h ESPs as well as spherical SPs were taken up by A549 cells. Both particle types did not induce any visible sign of cell damage compared to untreated control cells. To quantify the amount of particle uptake flow cytometry measurements were performed. Treatment of cells with increasing concentrations of SPs correlates with an increase in side-scatter (SS) values (ESI Fig. S15†). As SS values reflect the granularity of cells in general such an increase indicates uptake and/or adhesion of particles in/on the cells. Accordingly the number of cells with SS values above a certain threshold was quantified (“% cells SShigh”; for additional experimental details see the ESI Fig. S15†). Results from three independent experiments (Fig. 8D) revealed that approximately twice as many spherical SPs were taken up compared to ESPs (1.9 and 1.8 times more at concentrations of 3 μg mL−1 an d 10 μg mL−1, respectively). To exclude that different light scattering properties of the two particle populations rather than different uptake behavior lead to the observed shift in SS, cell pellets were further analyzed by ICP-OES to determine the total iron content in the cells. The results confirmed flow cytometry measurements revealing a twofold higher iron content in cells treated with spherical SPs (11.1 μg per 106 cells) compared to cells treated with ESPs (5.3 μg per 106 cells) (detailed description in the ESI†). Our results are in line with recent studies on polymer nanoparticle uptake into human mesenchymal stem cells expanding the confirmed negative dependency of cellular uptake on increased aspect ratios to colloidal SPs.11The observed difference between spherical and elongated SP can be explained by cells detecting only an apparent shape based on the geometry of the contact area. Elongated nanoparticles with their polar axis parallel to a cell present a big curvature radius. To describe results very similar to this work but for elongated polystyrene nanoparticles, Florez et al. developed a geometrical model according to which a 50 nm spherical nanoparticle that had been elongated by 100% would present the cell with an apparent curvature radius of up to 556 nm, thus, appearing as a much larger particle.11 The size of nanoparticles, however, has been well known to possess a strong effect on cellular uptake.45 Several pathways of internalization are available to nanoparticles, such as macropinocytosis and caveolin- or clathrin-mediated endocytosis, all of which, however, possess size limitations (120–150 nm for the latter). We hypothesize accordingly that the elongation of SPs leads to their detection by cells as much larger particles thereby making some pathways unavailable or at the least limiting their efficiency. For example, assuming perfect ellipsoid shape and an aspect ratio of 3, a 70 nm SP has an ESP counterpart of equal volume with a polar axis of 146 nm. The first spherical SP fits well within the range of caveolin- and clathrin-mediated endocytosis, whereas the ESPs polar axis is at the very limit even before considering the apparent curvature radius leaving mostly macropinocytosis as an available mechanism.
In summary, this first preliminary biological assessment revealed a shape-dependent cellular uptake without safety issues. These ellipsoid SPs offer an additional parameter, i.e. shape, to modulate interaction with cells for the rational design of nanosystems for biomedical application. For example ESPs may potentially be used for a shape-dependent “stealth effect” which has been postulated for high aspect ratio particles.13 Using different nanoparticle types as the building blocks could allow to adapt the ellipsoid SPs for different purposes and to test the influence of different chemical compositions. The influence of surface chemistry on protein adsorption and cellular uptake could be investigated and differences minimized, e.g. by additional coatings with poly(dopamine) or silica. A systematic approach beyond the herein presented initial proof of concept would be necessary to fully elucidate the possibilities and to give insight into the mechanisms of cell uptake.
4. Conclusions
In conclusion, we have presented a broadly applicable method for the fabrication of ellipsoid-shaped supraparticles from one (ESPs) or several (HESPs) types of inorganic nanoparticles in various ratios and combinations. The described method is highly versatile and modular, allowing a free choice of the inserted hydrophobic nanoparticle combinations. All employed nanoparticle compositions resulted in ellipsoid-shaped supraparticles with homogeneous distribution of the nanoparticle building blocks. Thus, optical, magnetic or catalytic properties can be unified in unprecedented supraparticles of ellipsoid shape. As an example, superparamagnetism, a functional property of high interest for magnetic manipulation and MRI, was introduced into the ellipsoid hybrid structure. Furthermore, a first time biological assessment of ellipsoid supraparticles revealed a pronounced reduction in cellular uptake compared to a spherical reference possibly resulting from a shape-dependent “stealth effect”. Our results strongly point towards the ellipsoid shape as an important rational design parameter for supraparticles. Additionally, the modular compositions open a tool box which can be adapted to different purposes including clinically driven applications. With a systematic approach to explore the power of the methodology beyond this proof of concept we expect to fabricate new anisotropically shaped and functional supraparticles for various applications especially in the biomedical field.Acknowledgements
We thank Prof. G. Jakob (University of Mainz) for the SQUID measurements, Dr A. Remhof for XRD data, Dr A. Schoth (Max Planck Institute for Polymer Research, Mainz) for donating silica nanoparticles, Prof. H. Frey (University of Mainz) for his support, E. Michel and K. Grieder (Empa) for ICP-OES measurements. Cordula Hirsch and Peter Wick were funded partly by the Competence Centre for Materials Science and Technology (CCMX) Project NanoScreen.Notes and references
- Z. D. Lu and Y. D. Yin, Chem. Soc. Rev., 2012, 41, 6874–6887 RSC.
- J. Guo, W. L. Yang and C. C. Wang, Adv. Mater., 2013, 25, 5196–5214 CrossRef CAS PubMed.
- T. Wang, D. LaMontagne, J. Lynch, J. Q. Zhuang and Y. C. Cao, Chem. Soc. Rev., 2013, 42, 2804–2823 RSC.
- Z. Lu, C. Gao, Q. Zhang, M. Chi, J. Y. Howe and Y. Yin, Nano Lett., 2011, 11, 3404–3412 CrossRef CAS PubMed.
- O. Chen, L. Riedemann, F. Etoc, H. Herrmann, M. Coppey, M. Barch, C. T. Farrar, J. Zhao, O. T. Bruns, H. Wei, P. Guo, J. Cui, R. Jensen, Y. Chen, D. K. Harris, J. M. Cordero, Z. W. Wang, A. Jasanoff, D. Fukumura, R. Reimer, M. Dahan, R. K. Jain and M. G. Bawendi, Nat. Commun., 2014, 5, 5093 CrossRef CAS PubMed.
- Z. D. Lu, M. M. Ye, N. Li, W. W. Zhong and Y. D. Yin, Angew. Chem., Int. Ed., 2010, 49, 1862–1866 CrossRef CAS PubMed.
- J. Jeong, E. K. Kwon, T. C. Cheong, H. Park, N. H. Cho and W. Kim, ACS Appl. Mater. Interfaces, 2014, 6, 5297–5307 CAS.
- J. Ge, H. Lee, L. He, J. Kim, Z. Lu, H. Kim, J. Goebl, S. Kwon and Y. Yin, J. Am. Chem. Soc., 2009, 131, 15687–15694 CrossRef CAS PubMed.
- Q. Zhang, J.-B. Joo, Z. Lu, M. Dahl, D. L. Oliveira, M. Ye and Y. Yin, Nano Res., 2011, 4, 103–114 CrossRef CAS.
- T. Bollhorst, S. Shahabi, K. Wörz, C. Petters, R. Dringen, M. Maas and K. Rezwan, Angew. Chem., Int. Ed., 2015, 54, 118–123 CrossRef CAS PubMed.
- L. Florez, C. Herrmann, J. M. Cramer, C. P. Hauser, K. Koynov, K. Landfester, D. Crespy and V. Mailänder, Small, 2012, 8, 2222–2230 CrossRef CAS PubMed.
- R. M. Fratila, S. Rivera-Fernandez and J. M. de la Fuente, Nanoscale, 2015, 8233–8260 RSC.
- X. P. Duan and Y. P. Li, Small, 2013, 9, 1521–1532 CrossRef CAS PubMed.
- R. P. Cowburn, J. Phys. D: Appl. Phys., 2000, 33, R1–R16 CrossRef CAS.
- I. Andreu, E. Natividad, L. Solozábal and O. Roubeau, ACS Nano, 2015, 9, 1408–1419 CrossRef CAS PubMed.
- M. Wang, L. He, Y. Hu and Y. Yin, J. Mater. Chem., 2013, 1, 6151–6156 CAS.
- S. Yang, C. Cao, Y. Sun, P. Huang, F. Wei and W. Song, Angew. Chem., Int. Ed., 2015, 2661–2664 CrossRef CAS PubMed.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS PubMed.
- Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664–670 CrossRef CAS PubMed.
- Z. J. Zhou, X. L. Zhu, D. J. Wu, Q. L. Chen, D. T. Huang, C. J. Sun, J. Y. Xin, K. Y. Ni and J. H. Gao, Chem. Mater., 2015, 27, 3505–3515 CrossRef CAS.
- M. Wang, L. He, W. Xu, X. Wang and Y. Yin, Angew. Chem., Int. Ed., 2015, 54, 7077–7081 CrossRef CAS PubMed.
- J. M. Haberl, A. Sánchez-Ferrer, A. M. Mihut, H. Dietsch, A. M. Hirt and R. Mezzenga, Adv. Mater., 2013, 25, 1787–1791 CrossRef CAS PubMed.
- M. B. Bannwarth, S. W. Kazer, S. Ulrich, G. Glasser, D. Crespy and K. Landfester, Angew. Chem., Int. Ed., 2013, 52, 10107–10111 CrossRef CAS PubMed.
- J. Zhou, L. Meng, X. Feng, X. Zhang and Q. Lu, Angew. Chem., Int. Ed., 2010, 49, 8476–8479 CrossRef CAS PubMed.
- C. Herrmann, M. B. Bannwarth, K. Landfester and D. Crespy, Macromol. Chem. Phys., 2012, 213, 829–838 CrossRef CAS.
- J. Zhuang, A. D. Shaller, J. Lynch, H. Wu, O. Chen, A. D. Q. Li and Y. C. Cao, J. Am. Chem. Soc., 2009, 131, 6084–6085 CrossRef CAS PubMed.
- T. Wang, X. R. Wang, D. LaMontagne, Z. L. Wang, Z. W. Wang and Y. C. Cao, J. Am. Chem. Soc., 2012, 134, 18225–18228 CrossRef CAS PubMed.
- M. B. Bannwarth, A. Camerlo, S. Ulrich, G. Jakob, G. Fortunato, R. M. Rossi and L. F. Boesel, Chem. Commun., 2015, 51, 3758–3761 RSC.
- J. K. Oh and J. M. Park, Prog. Polym. Sci., 2011, 36, 168–189 CrossRef CAS.
- A. Schoth, C. Wagner, L. L. Hecht, S. Winzen, R. Munoz-Espi, H. P. Schuchmann and K. Landfester, Colloid Polym. Sci., 2014, 292, 2427–2437 CAS.
- E. S. Reynolds, J. Cell Biol., 1963, 17, 208–212 CrossRef CAS PubMed.
- D. Hofmann, S. Tenzer, M. B. Bannwarth, C. Messerschmidt, S.-F. Glaser, H. Schild, K. Landfester and V. Mailaender, ACS Nano, 2014, 8, 10077–10088 CrossRef CAS PubMed.
- A. Burns, H. Ow and U. Wiesner, Chem. Soc. Rev., 2006, 35, 1028–1042 RSC.
- S. Widmer, M. J. Reber, P. Muller, C. E. Housecroft, E. C. Constable, R. M. Rossi, D. Bruhwiler, L. J. Scherer and L. F. Boesel, Analyst, 2015, 140, 5324–5334 RSC.
- T. Ribeiro, C. Baleizao and J. P. S. Farinha, Materials, 2014, 7, 3881–3900 CrossRef CAS.
- C. Herrmann, A. Turshatov and D. Crespy, ACS Macro Lett., 2012, 1, 907–909 CrossRef CAS.
- D. Li and Y. N. Xia, Adv. Mater., 2004, 16, 1151–1170 CrossRef CAS.
- A. Greiner and J. H. Wendorff, Angew. Chem., Int. Ed., 2007, 46, 5670–5703 CrossRef CAS PubMed.
- E. S. Medeiros, L. H. C. Mattoso, R. D. Offeman, D. F. Wood and W. J. Orts, Can. J. Chem., 2008, 86, 590–599 CrossRef.
- J. Pelipenko, J. Kristl, B. Jankovic, S. Baumgartner and P. Kocbek, Int. J. Pharm., 2013, 456, 125–134 CrossRef CAS PubMed.
- A. Camerlo, A. M. Bühlmann-Popa, C. Vebert-Nardin, R. Rossi and G. Fortunato, J. Mater. Sci., 2014, 49, 8154–8162 CrossRef CAS.
- M. B. Bannwarth, S. Utech, S. Ebert, D. A. Weitz, D. Crespy and K. Landfester, ACS Nano, 2015, 9, 2720–2728 CrossRef CAS PubMed.
- M. Rösslein, J. T. Elliott, M. Salit, E. J. Petersen, C. Hirsch, H. F. Krug and P. Wick, Chem. Res. Toxicol., 2015, 28, 21–30 CrossRef PubMed.
- H. Meng, S. Yang, Z. X. Li, T. Xia, J. Chen, Z. X. Ji, H. Y. Zhang, X. Wang, S. J. Lin, C. Huang, Z. H. Zhou, J. I. Zink and A. E. Nel, ACS Nano, 2011, 5, 4434–4447 CrossRef CAS PubMed.
- H. Kettiger, A. Schipanski, P. Wick and J. Huwyler, Int. J. Nanomed., 2013, 8, 3255–3269 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19861a |
| This journal is © The Royal Society of Chemistry 2016 |






