Dispersive solid phase extraction of gold with magnetite-graphene oxide prior to its determination via microwave plasma-atomic emission spectrometry†
Abstract
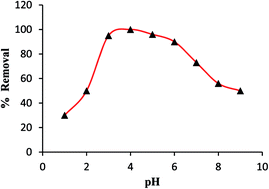
In this paper we demonstrated the quantitative analysis of gold in environmental water samples using microwave plasma atomic emission spectrometry (MP-AES) after dispersive solid phase extraction (DSPE) on magnetite graphene oxide (m-GO). The benefits of the MP-AES are related to cheap analysis costs and improved operational safety with nitrogen used for plasma generation. The detection limit, 3σ m−1, (0.005 μg L−1), inter and intra-day precision (2.6% and 3.2%) and dynamic range (0.02–500 μg L−1) for m-GO coupled MP-AES were compared and matrix effects were evaluated with respect to environmental samples. Recovery of (spiked) analytes ranges from 97.0 to 101%. The developed MP-AES method can offer a comparable or better performance to flame atomic absorption spectrometry and/or inductively coupled plasma optical emission spectrometry with respect to routine analysis for a regulatory program and may be applied as an active method.

 Please wait while we load your content...
Please wait while we load your content...