Oxidative dehydrogenation of thiols to disulfides at room temperature using silica supported iron oxide as an efficient solid catalyst†
Susmita Paul* and
S. M. Islam*
Department of Chemistry, University of Kalyani, Nadia, West Bengal, India. E-mail: susmitapaul2007@rediffmail.com; manir65@rediffmail.com; Fax: +91 33 2582 8282; Tel: +91 94 7419 7728 Tel: +91 33 2582 8750
First published on 16th September 2016
Abstract
Selective transformation of thiols to disulfides by means of oxidative dehydrogenation has been described using silica supported iron oxide under base- and solvent-free reaction conditions at room temperature in an open atmosphere. The easiness of catalyst preparation, green reaction conditions, easy separation of the formed products and catalyst from the reaction mixture, and recyclability of the catalyst, are the most attractive facets of our synthetic procedure which, being ecofriendly, will find immense applications in academic and industrial sectors.
Introduction
Molecular alteration is a prime dynamic force for chemical research and present approaches focus on the emergent awareness of the environmental impact of chemical production. The development of clean synthetic methodologies can open new doorways to gentle and cost-effective processes. Central aspects of research toward more sustainable processes for chemical synthesis involve replacement of heavy and toxic metal catalysts, organic solvents as reaction media, development of strategies to achieve higher atom economy, escaping from strong basic reaction conditions, and energy saving procedures.1 Oxidative dehydrogenation catalyzed by various metal oxides including iron oxides is well known in the literature and has attracted considerable attention over the years in the arena of industrial and academic research2 because naturally occurring iron oxides are ubiquitously found in the environment and are considered to be non-toxic to the environment in general. Iron oxides also find extensive usage in magnetic storage, medication, chemical industries and water refining. Supported or modified iron oxide catalyzed reactions are one of the attractive alternatives for green chemical synthesis3 because of the facilities associated with heterogeneous catalyst systems and environmental benefit of non-toxic iron oxide.4 Iron and iron oxides are abundant in nature and iron is one of the most versatile transition metals which act as important redox centres in biological systems and in many chemical transformations.5 On the other hand, disulfides are key intermediates in a broad range of organic syntheses. Organic compounds containing disulfides play crucial roles in chemistry and biology6 e.g. disulfide bond formation is important in peptides7 and bioactive molecules.8 Moreover, thiols can be conveniently protected as disulfides and can be regenerated by cleavage of the S–S bond.9 Due to the low bond energy of the sulfur–sulfur bond in disulfides, thiols can be easily over-oxidized giving thiosulfinates, thiosulfonates, and sulfonic acids and hence efforts are focused on their selective oxidation to disulfides without over-oxidation which has been exhaustively investigated over the years.10Among the several methods of preparing disulfides,11 the most straightforward methodology involves oxidative coupling of thiols to disulfides.12 In recent times, cleaner protocols have been described by A. Talla et al.12a under photocatalytic conditions using acetonitrile or ethanol as the solvent and an equivalent amount of TMEDA; and under solvent-free conditions by Montazerozohori et al.12f Despite significant efforts being made so far, most of these protocols suffer from use of expensive and/or lethal reagents, organic or inorganic bases, longer reaction times, strong oxidizing agents or flow of molecular oxygen.11,12 Among some approaches involving iron/iron oxide as heterogeneous catalysts for oxidative coupling of thiols to disulfides,12d H. Garcia et al. described Fe–BTC as a catalyst for the selective preparation of disulfides in acetonitrile solvent at 70 °C with the help of external oxygen flow. However, their method did work effectively in several cases; moderate yields (60–70%) were obtained with aliphatic thiols and with longer reaction time. A more recent approach using SBA-15 supported iron oxide nanoparticles for disulfide synthesis involves use of H2O2 in acetonitrile solvent at room temperature.12g However, the scope of this procedure is limited to fluorothiophenol as the reaction needs temperature 0 °C and inertness of aliphatic thiols to undergo self coupling or coupling with aromatic thiols to produce unsymmetrical disulfides. Thus a very careful survey of literature reports encourages us to invent a general and straightforward procedure for the synthesis of disulfides from thiols without over-oxidation following the principles of green chemistry. Herein, we report an efficient method for synthesizing disulfides from thiols using silica supported iron oxide catalyst at room temperature without the need of any solvent, external oxygen flow or the presence of highly oxidizing agents and bases (Scheme 1).
Results and discussion
In continuation of the search for better synthetic methodology using silica or silica supported iron catalysts,13 we have prepared silica supported iron oxide within a very short time which was then employed to establish a general procedure for conversion of thiols selectively and efficiently to corresponding disulfides for which 99% atom efficiency and a high TON/TOF (8000/1143 h−1; Table 1, entry 7) is achieved easily.| Entry | Catalyst | Amount of catalyst | Time | Conv.a (%) |
|---|---|---|---|---|
| a % conversions were checked by HPLC using MeOH as solvent.b Reaction carried out by using commercially available Fe2O3; Cat. A# (1 mmol Fe2O3 mixed with 1 g silica); Cat. B# (0.5 mmol Fe2O3 mixed with 1 g silica); Cat. C# (0.25 mmol Fe2O3 mixed with 1 g silica); Cat. D# (0.1 mmol Fe2O3 mixed with 1 g silica). | ||||
| 1 | Nil | Nil | 24 h | 5 |
| 2 | Silica | 250 mg | 24 h | 7 |
| 3 | Cat. A | 250 mg | 40 min | 100 |
| 4 | Cat. B | 500 mg | 50 min | 100 |
| 5 | Cat. B | 250 mg | 1 h | 100 |
| 6 | Cat. C | 250 mg | 2 h 30 min | 100 |
| 7 | Cat. D | 250 mg | 7 h | 100 |
| 8 | Cat. A# | 250 mg | 50 min | 100 |
| 9 | Cat. B# | 250 mg | 1 h 20 min | 100b |
| 10 | Cat. C# | 250 mg | 2 h 40 min | 100 |
| 11 | Cat. D# | 250 mg | 9 h | 100 |
| 12 | Fe2O3 | 20 mg | 30 h | 85 |
We have prepared a series of catalysts by varying the addition of an accurately weighed amount of ferric chloride to 1 g of silica (MERK, silica gel G for TLC) in a 100 mL round bottomed flask and then 6 mL acetone is added to the flask. The acetone is then evaporated under continuous stirring of the mixture at 35 °C. After complete evaporation of the acetone, the flask (containing a yellow solid mass) is heated at 250 °C for three hours in an oil bath under continuous magnetic stirring in an open atmosphere. The yellow solid mass turns to a deep amber colour. The solid silica supported iron oxide catalyst thus prepared is then employed for selective transformation of thiols to disulfides. The series of catalysts with decreasing iron content is designated as: catalyst A (prepared by mixing 1 mmol FeCl3 with 1 g silica gel G); catalyst B (prepared by mixing 0.5 mmol FeCl3 with 1 g silica); catalyst C (prepared by mixing 0.25 mmol FeCl3 with 1 g silica); catalyst D (prepared by mixing 0.1 mmol FeCl3 with 1 g silica).
The silica supported iron oxide prepared by the thermal decomposition of ferric chloride is then characterized by solid state UV, FT-IR spectroscopy, TGA analysis and SEM analysis.
DRS-UV analysis in the range of 200–800 nm shows a distinct difference between the silica gel G and catalyst B. Catalyst B shows three absorbance peaks viz. a sharp peak around 250 nm, another peak around 350 nm, and a broad peak between 500–600 nm (Fig. 1b) whereas silica gel G does not show any characteristic peaks in this region (Fig. 1a).
The FT-IR study of silica gel G (Fig. 2a) and catalyst B (Fig. 2b) does not show much distinctive difference between the spectra, which both contain O–H stretching at 3400 cm−1, and at 582 cm−1 for Si–O stretching.
The thermal stability of the catalyst B in comparison to the support silica gel G is examined by TG/DTA analysis (Fig. 3). The study is carried out in the range of 25–500 °C with a 10 °C min−1 heating rate. The initial steady decrease of the line graph of the TG/DTA spectra of silica gel G (Fig. 3a) and catalyst B (Fig. 3b) may be due to the loss of water present in the silica matrix, and the absence of any sharp changes in the range of 200–250 °C for catalyst B (Fig. 3b) signifies the continuity of the same phase present in the catalyst system B.
SEM analysis of catalyst B is performed and the pictures are given in Fig. 4 (plots are included in the ESI†).
We start our study of synthesizing disulfides from thiols by taking thiophenol as the model substrate to optimize the reaction conditions. The results are summarized in Table 1. We have observed the formation of disulfide only in trace amounts under neat conditions without any catalyst or support, or in the presence of a support without catalyst (Table 1, entry 1 and 2 respectively). The reaction proceeds to full conversion to disulfide with 250 mg of catalyst A within a very short reaction time without any requirement of base or solvent (Table 1, entry 3). The time needed for complete conversion increases with decreased catalyst loading as expected (Table 1, entries 3–7). The fact that the silica supported iron oxide catalyst (prepared by thermal pyrolysis of FeCl3) shows comparable activity14 with commercially available iron oxide (mixed by mechanical grinding in a mortar and pestle with silica (MERK, silica gel G for TLC)) (Table 1, entries 8–11) emphasizes that the Fe2O3 moiety is the active catalytic phase in our catalyst system. The importance of silica as a support13 in this study is revealed by the fact that 85% conversion of thiophenol to corresponding disulfide requires as long as 30 h when we employ commercially available iron oxide in the reaction under neat conditions (Table 1, entry 12), while our system took only 1 h for complete conversion (Table 1, entry 5). This result may be due to the coating of iron oxide particles by the formed diphenyl disulfide thereby inhibiting the chances of showing any further catalytic activity under neat catalytic conditions. However the situation is different for the silica supported iron oxide system where the formed diphenyl disulfide gets distributed over a large surface area provided by the mesoporous support and the catalytically active centres remain free for further reaction. These amazing observations highlight the importance of silica as an indispensable support for this reaction. With the optimization data in our hands, we have chosen the reaction with 250 mg of the supported catalyst B (0.5 mmol FeCl3 supported on 1 g silica) to convert 2 mmol of the thiophenol to corresponding disulfide as the model one. Table 2 clearly shows the efficiency and cleanliness of our procedure in comparison to some recently developed methodologies for preparing disulfides from thiols by a photocatalytic method without metal catalyst (Table 2, entry 1), a solid state method (Table 2, entry 2) and with iron catalyzed systems (Table 2, entries 3–7).
| Entry | Catalyst/oxidant | Catalyst/oxidant amount | Additive | Solvent | RSH | Temp. | Time | Yield % | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Eosin Y | 1 mol% | TMEDA (1 equiv. occasional adding) 24 W CFL, air | Ethanol | Aromatic, heteroaromatic, aliphatic | Not mentioned | 16 h | 93–99 | 12a |
| 2 | Moist sodium periodate | 1 mmol | — | — | Aromatic, heteroaromatic, benzyl, aliphatic | RT | (1.5–5) min | 70–96 | 12f |
| 3 | Fe(BTC)/external oxygen flow | 100 mg | — | Acetonitrile (4 mL) | Aromatic, heteroaromatic, benzyl, aliphatic | 70 °C | (1–20) h | 55–91 | Cross ref. no. 45 of ref. 11a |
| 4 | Fe-NPS@SBA-15/H2O2 | 1 mol% | — | Acetonitrile (3 mL) | Aromatic, heteroaromatic, benzyl | 25 °C | (15–360) min | 70–99 | 12g |
| 5 | Fe(TPP)Cl/UHP | 0.02 mol/1 mmol | — | Methanol (10 mL) | Aromatic, heteroaromatic, benzyl | 0 °C | (10–90) min | 60–91 | 12e |
| 6 | Nano Fe2O3 | 80 mg | Ionic liquid | Methanol | Aromatic, aliphatic | 60 °C | (10–75) min | 45–99 | 12i |
| 7 | Cobalt–iron magnetic composites | 0.1 wt% | — | DMF | Aromatic | (25–50) °C | (0.5–3) h | 100 | 12h |
| 8 | Silica supported iron oxide | 250 mg (0.1 mol%) | — | — | Aromatic, heteroaromatic, benzyl, aliphatic | (27–30) °C | (1–8) h | (72–95) | Our procedure |
We explore the scopes and limitations of our reaction protocol by employing different thiols and the observations are summarized in Table 3. Ortho- and para-substituted aromatic thiophenols including heteroaromatic thiol (pyridyl) result in very good yields of the corresponding disulfides exclusively (82–95%) within a very short reaction time (1–5) h (Table 3, entries 1–5 and 15). The methoxy substituted thiophenols (both ortho and para) require comparatively longer reaction time (8 h) to produce very good yields of disulfides (Table 3, entries 6 and 7). Halogenated thiols also produce very good yields within a reasonable time period (Table 3, entries 8 and 9). Alicyclic thiol viz. cyclohexylthiol, and other aliphatic straight chain thiols, namely pentane and heptane thiol, also produce excellent yields of the corresponding disulfides (Table 3, entries 11–13). We have isolated disulfide of thioglycolic acid in very good yield and in this case the product has been separated after esterification of the carboxylic group with ethanol in an one pot reaction procedure. Here, worth mentioning is that Garcia et al. reported only good yields of the disulfides in the case of heteroaromatic thiols, moderate yield with benzyl thiol and low yield with thioacetic acid.
| Entry | Thiol | Time | Yielda (%) |
|---|---|---|---|
| a % yield represents yield after column chromatographic purification however, solid disulfides could be obtained by simple filtration followed by evaporation of the filtrate.b Disulfide was isolated by esterification of the acid by ethanol after completion of the reaction in a one pot procedure. | |||
| 1 |  |
1 h | 95 |
| 2 |  |
3 h | 92 |
| 3 |  |
2.5 h | 93 |
| 4 |  |
8 h | 72 |
| 5 |  |
3 h | 92 |
| 6 | 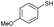 |
8 h | 82 |
| 7 |  |
8 h | 83 |
| 8 |  |
3 h | 86 |
| 9 |  |
5 h | 87 |
| 10 |  |
2 h | 86 |
| 11 |  |
3 h | 85 |
| 12 |  |
5 h | 88 |
| 13 |  |
7 h | 85 |
| 14 |  |
8 h | 83b |
| 15 | 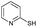 |
8 h | 82 |
Depending on the fact that iron oxide can be involved in redox reactions in heterogeneous conditions or on a solid surface,14,15 we propose the plausible mechanism for oxidative dehydrogenation as depicted in Scheme 2. In the first step the acidic hydrogen of –SH gets attached to one of the doubly bonded oxygen atoms of iron oxide through the lone pair of electrons thereby weakening the strength of the –S–H bond [E]. The electron deficiency thereby formed on the iron centre can be partially met by the electron donating capability of the sulphur in the thiophenol group [F] which causes further weakening of the –S–H bond. In the next step the S atom of another thiol attacks the S atom of the catalytically bonded thiol [G] which ultimately forms the desired disulfide bond through [H]. The blue colour signifies a developing bond and red signifies a breaking bond. In this reaction sequence iron oxide gets partially reduced to –FeOH which gets converted to iron oxide again by aerial oxygen.
 | ||
| Scheme 2 Probable mechanism for aerobic oxidation of thiols to disulfides using silica supported iron oxide. | ||
The advantage of silica supported iron oxide in this reaction is examined by studying its recovery and reuse after the first run (Table 4) taking thiophenol as the reactant. After completion of the reaction, the solid mixture is centrifuged repeatedly with dicholoromethane (2 mL × 3). The dicholoromethane extract is examined under AAS which shows bare existence of leached iron. The solid is then dried in a hot air oven at 100 °C for 2 h and then the next batch of reaction is performed with the recovered silica-supported iron oxide and found to be equally active in catalyzing the process. Table 3 shows the results of six consecutive recycle runs. The DRS-UV analysis and FT-IR spectra of catalyst B after six recycle runs do not show differences in spectral pattern (Fig. 5a and b respectively) and SEM analysis performed on the reused catalyst B (Fig. 5c) shows that the catalyst loses granularity in comparison to the fresh catalyst (Fig. 4). The catalyst is air stable and can be used up to six months after preparation. The reaction is scaled up to 10 mmol of thiophenol with 250 mg of catalyst B which took 38 h for 100% conversion to diphenyl disulfide.
 | ||
| Fig. 5 (a) DRS-UV analysis of catalyst B after six runs. (b) FT-IR analysis of catalyst B after six recycle runs. (c) SEM analysis of catalyst B after the sixth cycle. | ||
Conclusions
In conclusion, atmospheric oxidation of thiols to disulfides can be performed using silica supported iron oxide as the reusable catalyst under very mild reaction conditions. The catalyst is effectively applicable for a very wide range of thiols including aromatic, aliphatic, alicyclic, benzylic and heteroaromatic thiols and easy separation of the reaction product from the reaction mixture by only simple filtration makes the procedure economically more viable than that demonstrated by Fe(BTC) or SBA 15 supported iron oxide catalyst.12d,g The reaction protocol does not require any extra flow of oxygen or highly oxidizing agent such as hydrogen peroxide or sodium periodate; the conditions do not require the presence of strong inorganic bases or organic bases like TMEDA. The catalyst precursor FeCl3 is very cheap and the final catalyst is environmentally non-hazardous. This protocol, being eco-friendly, will find immense industrial and academic applications. Further research to give more insight into this reaction mechanism is underway in our laboratory. The discovery that disulfide bonds can be prepared under solvent free conditions on an oxide support at room temperature could open a new window to investigate the mechanism of biological evolution processes where disulfide bond formation plays a key role in peptide synthesis and in determining the tertiary and quaternary structure of proteins.Experimental
Representative procedure for the synthesis of di-phenyl-di-sulfide
Silica supported iron oxide (catalyst B, 250 mg) was taken in a glass mortar and then 2 mmol of thiophenol was added to the catalyst and mixed by a pestle. After mixing the solid mass was transferred to a 25 mL round bottomed flask fitted with a guard tube and a small magnet was introduced for continuous magnetic stirring for 1 h at room temperature. After the completion of the reaction (checked by TLC) the reaction mixture was washed successively with dichloromethane (2 mL × 3) and filtered through Whatmann 42 filter paper. The filtrate was then evaporated to obtain white crystals of di-phenyl di-sulfide. The product was then characterized by 1H and13C NMR spectroscopy and the melting point was compared with the literature value.Table 2, entry 1: 1H NMR (CDCl3, d ppm−1 relative to TMS, 300 MHz): 7.18–7.31 (6H, m); 7.48–7.50 (4H, d, J = 7.5 Hz); 13C NMR (CDCl3, d ppm−1, 75 MHz): 127.1, 127.4, 129, 136.9. Melting point observed 60 °C [(59–62) °C (lit.)].
The representative procedure for the synthesis of diphenyl disulfide is followed for the preparation of other disulfides mentioned in Table 3.
Acknowledgements
Susmita Paul thankfully acknowledges UGC DS Kothari funding agency for financial support (No. F.4-2/2006/(BSR)/CH/13-14/0075); Prof. Anilava Kabiraj, Department of Zoology, University of Kalyani for recording AAS spectra, Mominul Islam and Mita Halder for recording spectroscopic data; SMI acknowledges Department of Science and Technology, New Delhi, Govt. of India, (DST-SERB), University grant Commission, New Delhi, Govt. of India, (UGC), and Department of Science and Technology, Govt. of West Bengal (DST-W.B.) for financial support. We gratefully acknowledge DST and UGC Govt. of India for providing support to the Department of Chemistry, University of Kalyani under FIST, PURSE and SAP programme.Notes and references
- P. T. Anastas and J. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 CrossRef CAS PubMed; D. Adam, Nature, 2000, 407, 938 CrossRef CAS PubMed.
- (a) B. Basu, S. Satapathy and A. K. Bhatnagar, Catal. Rev., 1993, 35, 571 CrossRef CAS; (b) J. J. H. B. Sattler, J. Ruiz-Martinez, E. Santillan-Jimenez and B. M. Weckhuysen, Chem. Rev., 2014, 114, 10613 CrossRef CAS PubMed; (c) E. H. Lee, Catal. Rev., 1974, 8, 285 CrossRef.
- (a) S. R. Pouran, A. A. A. Raman and W. M. A. W. Daud, J. Cleaner Prod., 2014, 64, 24 CrossRef; (b) A. Kanazawa, K. Satoh and M. Kamigaito, Macromolecules, 2011, 44, 1927 CrossRef CAS; (c) F. Adam, J. Andas and I. A. Rahman, Chem. Eng. J., 2010, 658 CrossRef CAS; (d) A. R. Hajipour and G. Azizi, Green Chem., 2013, 15, 1030 RSC; (e) K. Swapna, A. V. Kumar, V. P. Reddy and K. R. Rao, J. Org. Chem., 2009, 74, 7514 CrossRef CAS PubMed.
- (a) S. Laurent, D. Forge, M. Port, A. Roch, C. L. V. Robic and R. N. ElstMuller, Chem. Rev., 2008, 108, 2064 CrossRef CAS PubMed, and the references cited therein; (b) J. Mondal, T. Sen and A. Bhaumik, Dalton Trans., 2012, 41, 6173 RSC; (c) D. A. Alonso, C. Najera, I. M. Pastor and M. Yus, Chem. Euro. J., 2010, 16, 5274 CrossRef CAS PubMed.
- C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 104, 6217 CrossRef CAS PubMed.
- (a) H. Shinkai, K. Maeda, T. Yamasaki, H. Okamoto and I. Uchida, J. Med. Chem., 2000, 43, 3566 CrossRef CAS PubMed; (b) I. Hamachi, T. Nagase and S. Shinkai, J. Am. Chem. Soc., 2000, 122, 12063 CrossRef; (c) D. P. Gamblin, P. Garnier, S. van Kasteren, N. J. Oldham, A. J. Fairbanks and B. G. Davis, Angew. Chem., Int. Ed., 2004, 43, 828 CrossRef CAS PubMed; (d) J. Winum, M. Rami, A. Scozzafava, J. Montero and C. Supuran, Med. Res. Rev., 2008, 28, 445 CrossRef CAS PubMed; (e) A. Atkinson and D. R. Winge, Chem. Rev., 2009, 109, 4708 CrossRef CAS PubMed.
- M. Bodanszky, Principles of Peptide Synthesis, Springer, Berlin, 1984, ch. 4 Search PubMed.
- J. R. Johnson, W. F. Bruce and J. D. Dutcher, J. Am. Chem. Soc., 1943, 65, 2005 CrossRef CAS.
- (a) A. Ogawa, Y. Nishiyama, N. Kambe, S. Murai and N. Sonoda, Tetrahedron Lett., 1987, 28, 3271 CrossRef CAS, and references cited therein; (b) S. N. Maiti, P. Spevak, M. P. Singh, R. G. Micetich and A. V. N. Reddy, Synth. Commun., 1988, 18, 575 CrossRef CAS.
- S. Oae, D. Fukushima and Y. H. Kim, J. Chem. Soc., Chem. Commun., 1977, 407 RSC.
- (a) B. Mandal and B. Basu, RSC Adv., 2014, 4, 13854 RSC; (b) I. Shcherbakova and A. F. Pozharskii, in Comprehensive Organic Functional Group Transformations II, ed. A. R. Katritzky, R. Taylor and Ch. Ramsden, Pergamon, Oxford, 2004, vol. 2, pp. 210–233 Search PubMed; (c) P. C. Bulman Page, R. D. Wilkes and D. Reynolds, in Comprehensive Organic Functional Group Transformations, ed. A. R. Katritzky, O. Meth-Cohn and C. W. Rees, Pergamon, Oxford, 1995, vol. 2, pp. 177–187 Search PubMed; (d) R. Sato and T. Kimura, in Science of Synthesis, ed. N. Kambe, J. Drabowiczand and G. A. Molander, Thieme, Stuttgart–New York, 2007, vol. 39, pp. 573–588 Search PubMed; (e) D. Witt, Synthesis, 2008, 2491 CrossRef CAS.
- (a) A. Talla, B. Driessen, N. J. W. Straathof, L.-G. Milroy, L. Brunsveld, V. Hessel and T. Noël, Adv. Synth. Catal., 2015, 357, 2180 CrossRef CAS; (b) H. Firouzabadi, N. Iranpoor and M. A. Zolgol, Synth. Commun., 1998, 280, 1179 CrossRef; (c) K. Tanaka and K. Ajiki, Tetrahedron Lett., 2004, 45, 25 CrossRef CAS; (d) Cross ref. no. 41, 43–57, 59–62, 63, 65–70 of ref. 11a; (e) B. Karami, M. Montazerozohori, M. Moghadam, M. H. Habibi and K. Niknam, Turk. J. Chem., 2005, 29, 539 CAS; (f) M. Montazerozohori, S. Joohari, B. Karami and N. Haghighat, Molecules, 2007, 12, 694 CrossRef CAS PubMed; (g) F. Rajabi, T. Kakeshpour and M. R. Saidi, Catal. Commun., 2013, 40, 13 CrossRef CAS; (h) L. Menini, M. C. Pereira, A. C. Ferreira, J. D. Fabris and E. V. Gusevskaya, Appl. Catal., A, 2011, 392, 151 CrossRef CAS; (i) A. Sabet, A. Fakhraee, M. Ramezanpour and N. Alipour, International Scholarly and Scientific Research & Innovation, International Journal of Chemical, Molecular, Nuclear, Materials and Metallurgical Engineering, 2014, 8, 1184 Search PubMed.
- (a) S. Paul and B. Basu, Tetrahedron Lett., 2012, 53, 4130 CrossRef CAS, and references cited therein; (b) B. Basu, S. Paul and A. K. Nanda, Green Chem., 2009, 11, 1115 RSC; (c) B. Basu, S. Paul and A. K. Nanda, Green Chem., 2010, 12, 767 RSC; (d) B. Basu and S. Paul, Appl. Organomet. Chem., 2013, 27, 588 CAS.
- G. A. Bukhtiyarovaa, I. V. Deliia, N. S. Sakaevab, V. V. Kaicheva, L. M. Plyasovaa and V. I. Bukhtiyarova, Catal. Lett., 2007, 92, 89 Search PubMed.
- (a) H. Wu, J.-J. Yin, W. G. Wamer, M. Zeng and Y. Martin Lo, J. Food Drug Anal., 2014, 22, 86 CrossRef CAS PubMed; (b) H. Guo and A. S. Barnard, J. Mater. Chem. A, 2013, 1, 27 RSC; (c) W. M. C. R. C. Haynes, Handbook of chemistry and physics, Taylor & Francis, New York, 94th edn, 2013 Search PubMed; (d) P. M. Wood, Biochem. J., 1988, 253, 287 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV, IR, TGA, SEM analysis of support and catalyst, and scanned copy of 1H and 13C NMR spectra of selected disulfides. See DOI: 10.1039/c6ra19832e |
| This journal is © The Royal Society of Chemistry 2016 |





