Tannic acid stabilized silver nanoparticles for inkjet printing of conductive flexible electronics†
Abstract
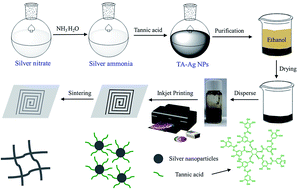
In this study, we report a simple, environmentally-friendly, and economically scalable approach to synthesize silver nanoparticles (Ag NPs) which were prepared as conductive inks for fabricating electrically conductive patterns by direct inkjet printing. Using tannic acid (TA) as a reducing agent and capping agent simultaneously, the whole reaction was conducted in aqueous solution at room temperature in a very short time. The obtained TA-Ag NPs could be collected by precipitation and kept in solid form and stored without oxidation for several months, which greatly simplified the preparation steps and is quite beneficial for the storage and transportation. The as-prepared TA-Ag NPs have an average diameter of 15 nm and could be well dispersed in water to form a stable and homogenous silver ink. A conductive pattern can be achieved by inkjet printing the inks using a common color printer. The influence of the sintering temperature and print layers on the conductive performance of the printed silver pattern was investigated in detail and the sheet resistance of printed pattern decreased to 2 Ω □−1 at a sintering temperature of 200 °C. The printed silver pattern shows good adhesion towards the paper substrate under tape test. With the advantages of low cost, simple and green preparation, mass production, and high conductivity, this proposed method has great potential for application in printing flexible electronics, as demonstrated by the inkjet printing of conductive LED device circuits.

 Please wait while we load your content...
Please wait while we load your content...