Tellurium-containing nanoparticles for controlled delivery of cisplatin based on coordination interaction†
Wei Cao,
Feng Li,
Ruofan Chen and
Huaping Xu*
Key Lab of Organic Optoelectronics and Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: xuhuaping@mail.tsinghua.edu.cn
First published on 26th September 2016
Abstract
Tellurium containing nanoparticles were designed and synthesized for the delivery of cisplatin based on coordination interaction. The tellurium containing nanomedicine could achieve better in vivo efficacy and reduce side effects, owing to the prolonged blood circulation. This kind of approach marked a new frontier of tellurium containing nanomedicine.
Advanced nanoscale systems have recently received enormous attention for drug delivery, especially for nanomedicine.1–5 Encapsulating free drugs in nanoparticles is desirable as nanoparticles can passively target cancer cells by the enhanced permeability and retention (EPR) effect.6–9 Compared to free drug molecules, nanomedicine could prolong the drug half-life, reduce side effects and enhance therapeutic efficacy.10–12 Passive-targeting nanoparticles reached clinical trial in 1980s, and later therapeutic nanocarriers on the basis of this strategy were approved for wider use.13–15 Numerous loading strategies have been reported so far, including hydrophobic interactions,16,17 chemical conjugation,18–20 electrostatic interactions21–25 and so on.26,27 Different loading interactions lead to different delivery behavior and drug efficacy. In this regard, developing delivery vehicles with specific loading interactions is still highly demanding.
Tellurium is an element with great biological potency and potential but has long appeared almost invisible in biology.28–30 Looking back at the history of chemistry, tellurium was actually discovered 35 years earlier than selenium, which is located right above the chalcogen group in the periodic table of elements. Tellurium containing compound was reported to catalyze the decomposition of peroxides.31,32 Currently some tellurium containing drug is under phase II clinical trial.33 While selenium-containing polymers have been shown to possess unique properties as multi-responsive materials for bionanotechnology,34–38 tellurium-containing polymers reported in recent years also demonstrate great potentials as innovative promising biomaterials.39–41 Tellurium-containing polymers could work as controlled delivery vehicle for cisplatin with decent loading capacity due to the coordination interactions between cisplatin and tellurium.18,19 Controlled release of different kinetics could be triggered by the competitive coordination of bio ligands.42,43 However, little has been known about the toxicity and the in vivo performance of tellurium containing nanomedicines.
In this study, an amphiphilic tellurium-containing compound was designed and synthesized for the delivery of cisplatin based on the coordination interactions (Scheme 1). Cisplatin was chosen as the model drug as it is one of the most successful drugs in clinical anticancer therapy. Despite its various advantages as the mainstream anticancer drugs nowadays, cisplatin has side effects that are hindering its clinical application, including kidney toxicity, bone marrow suppression, anaphylaxis etc. As shown in Fig. 1, tellurium was located in the hydrophobic center of TeCOOEG, while triethylene glycol was attached as the hydrophilic part. Compared with polymer materials, small molecules are easy to purify and have well-defined chemical structure. Therefore, small molecules could be well characterized and more likely to be approved by FDA. Through the coordination bond between platinum and tellurium, TeCOOEG could load cisplatin as the previously reported tellurium-containing polymer. The coordination interaction was first confirmed by NMR data. In 125Te NMR spectra as shown in Fig. 1b, the 125Te chemical shift of TeCOOEG was 232.6 ppm, and downshifted to 398.2 ppm after adding cisplatin. In accordance, the chemical shift for α proton of tellurium atom also shifted from 2.64 to 2.83 ppm (see ESI Fig. S5†). These obvious downshifts could be interpreted by the deshielding effect of Pt2+ cation. Secondly, XPS results provided more evidence (Fig. 1c and d). The Pt peaks for cisplatin shifted from 75.99 eV and 72.63 eV to 75.85 eV and 72.57 eV after coordination, which was in consistence with the trend in selenium-cisplatin coordination complex reported previously.44 While the Te peaks of TeCOOEG were 584.56 eV and 574.04 eV, after cisplatin loading, they changed to 584.56 eV and 574.04 eV, respectively. The XPS results indicated the existence of coordination interaction. Furthermore, ESI-Mass spectrum was conducted to analyze the coordination complex structure. The highest peak was found at 2349.86 (see ESI Fig. S6†), in agreement with the molecular formula of [Pt(TeCOOEG)3Cl]+, which indicated a maximum coordination ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
 | ||
| Scheme 1 Schematic illustration of the tellurium-containing nanoparticles for controlled delivery of cisplatin based on coordination interaction. | ||
The self-assembly behaviors before and after loading of cisplatin were studied in details. As the hydrophobic tellurium and the alkyl chains were connected to hydrophilic OEG chains on both sides, the amphiphilic TeCOOEG molecule could self-assemble to aggregates in water through the driving force of hydrophobic interactions. The dynamic light scattering (DLS) measurement of this assembly yielded the Critical Aggregate Concentration (CAC) at 4.0 × 10−6 M (Fig. 2a) and diameter at about 120 nm (see ESI Fig. S7†). The Transmission Electron Microscopy (TEM) image of the TeCOOEG assembly presents an approximately spherical morphology, with a size consistent with the DLS diameter result (Fig. 2c). The CAC of the tellurium–Platinum complex was also investigated through DLS measurement to be 3.2 × 10−5 M (Fig. 2b), which was 8 times that of TeCOOEG. The dramatic increase of CAC after complexion is in agreement with the fact that TeCOOEG is more hydrophobic, as it formed a milky solution while the Te/Pt complex formed a yellow transparent solution at the same concentration around 2.0 × 10−3 M. The improved hydrophilicity of Te/Pt complex might be attributed to the strengthened electrostatic interactions brought about by Pt2+ cation. And the TEM and DLS results both gave a diameter of around 140 nm, slightly larger than the TeCOOEG assembly. The size of nanomedicine determines its in vivo penetrability, accumulation and circulation to some extent, and this size, considered within the generally expected range of appropriate size, may enable the cisplatin-loaded nanoparticles as practical vehicles for cancer therapy.
Prior to in vivo studies, the anticancer activity of the cisplatin-loaded nanoparticles was examined through in vitro experiments. In vitro cytotoxicity of TeCOOEG and cisplatin-loaded nanoparticles was tested on HepG2 cells (human liver carcinoma cells). As shown in Fig. 3, TeCOOEG owned relatively good biocompatibility. The cancer cells incubated with cisplatin-loaded nanoparticles showed a much lower viability at the same concentration of Te, which proved the strengthened anticancer activity after coordination. Fluorescent micrograph study based on dichlorofluorescein diacetate (DCFH-DA), which could be quickly oxidized to fluorescent state by intracellular ROS, revealed that cisplatin loaded nanoparticles could generate ROS in large amount (see ESI Fig. S9†). While the cisplatin control group showed different ROS level, in which only much weaker fluorescence was observed. As overproduction of ROS could cause oxidative damage to lipids, DNA, proteins and lead to extensive cell damage and eventually cell death, this phenomenon observed here indicated that the nanoparticles have ROS-mediated anticancer activity.
To examine the safety and efficacy of the cisplatin-loaded nanoparticles, three proof-of-concept in vivo experiments were carried out. The antitumor activity, side effects and blood circulation time were studied respectively.
The antitumor activity in vivo was assessed on BALB/c nu/nu mice with HepG2 subcutaneous xenografts through intravenous (i.v.) injection. Tumor volumes were measured over time and taken out after 4 weeks for each group. Cisplatin-loaded nanoparticles were able to obtain a comparable result with free cisplatin, tightly controlling the tumor growth through the 4 weeks. While in the control group without treatment, the tumors growth was accelerated since the third week. These in vivo results indicated that the load of cisplatin through coordination interactions could control the tumor growth and provide an effective anticancer therapy (Fig. 4).
In vivo side effects were studied on healthy BALB/c mice with normal immunity. Three experiment groups were set, treated with cisplatin and cisplatin-loaded nanoparticles respectively, along with the saline control group. In our experiments, the nanoparticles could significantly reduce the side effects caused by cisplatin. First, the mice treated with three cisplatin injections showed obvious body weight loss, as shown in Fig. 5a. While cisplatin-loaded nanoparticles showed less of that. Then, renal toxicity was also assessed by measuring the amounts of creatinine and uric acid in the kidney through serum biochemical tests. Both creatinine and uric acid were significantly elevated by cisplatin (see ESI Fig. S10†), while almost no difference between cisplatin-loaded nanoparticles group and control was observed. The self-assembled nanostructure could also help escape the filtration by kidney, which may lead to fewer side effects on kidney. In addition, a distinguishable decline of the platelet (PTL) was observed in the cisplatin group (see ESI Fig. S11†), which is a sign of dysfunction of coagulation ability. In contrast, the cisplatin-loaded nanoparticles group could maintain a normal level of PTL. Given these results we considered this kind of tellurium containing nanomedicine to be able to diminish those intrinsic side effects of cisplatin.
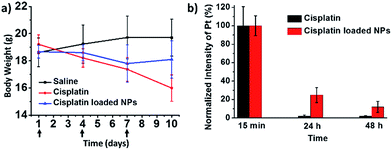 | ||
| Fig. 5 In vivo side effects on healthy BALB/c mice. (a) Body weight plot after three i.v. injections. (b) Plasma clearance after one i.v. injection. | ||
The incorporation of cisplatin into nanoparticles through coordination interactions is a substantial advantage to attain prolonged blood circulation and may eventually lead to enhanced efficacy. Experiment on plasma clearance of platinum after intravenous injection was conducted for verification. As shown in Fig. 5b, for cisplatin-loaded nanoparticles, the Pt content in bloodstream was about 25% after 24 hours, and after another 24 hours over 10% Pt is still retained. On the contrary, the Pt content of cisplatin group was almost cleared 24 hours after injection. The hydrophilic tri(ethylene glycol) shell might account for the improved blood circulation time by prevent macrophage clearance. This prolonged Pt circulation may help to increase drug accumulation at a target site and eventually lead to better anticancer effect and few side effects through EPR effects.
Conclusions
In summary, we have demonstrated a successful example of utilizing the tellurium-containing compound as delivery vehicle of cisplatin with enhanced efficacy. Cisplatin could be loaded in the nanoparticles through coordination interaction between tellurium and platinum. Better efficacy could be achieved with ROS mediated anticancer activity. This kind of approach could reduce side effects of cisplatin in animal study. Our results help to elucidate the in vivo performance of tellurium-containing nanomedicine. It marks a new frontier by providing a glimpse into the future of the tellurium-containing nanomedicine for more efficient drug delivery. Further rational design in chemical structure may lead to well-defined nanostructure and better targeting effect.Acknowledgements
This work was financially supported by the National Basic Research Program of China (2013CB834502), National Science Foundation for Distinguished Young Scholars (21425416), the National Natural Science Foundation of China (91427301), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (21421064). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Tsinghua University with assurance number #A5916-01, and all efforts were made to minimize animal suffering.Notes and references
- D. Peer, J. M. Karp, S. Hong, O. C. FaroKhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- K. J. Cho, X. Wang, S. M. Nie, Z. Chen and D. M. Shin, Clin. Cancer Res., 2008, 14, 1310–1316 CrossRef CAS PubMed.
- M. B. Zakaria, A. A. Belik, C.-H. Liu, H.-Y. Hsieh, Y.-T. Liao, V. Malgras, Y. Yamauchi and K. C. W. Wu, Chem.–Asian J., 2015, 10, 1457–1462 CrossRef CAS PubMed.
- K. C. W. Wu, Y. Yamauchi, C.-Y. Hong, Y.-H. Yang, Y.-H. Liang, T. Funatsu and M. Tsunoda, Chem. Commun., 2011, 47, 5232–5234 RSC.
- Q. Feng, J. Sun and X. Jiang, Nanoscale, 2016, 8, 12430–12443 RSC.
- H. Sun, F. Meng, R. Cheng, C. Deng and Z. Zhong, Expert Opin. Drug Delivery, 2013, 10, 1109–1122 CrossRef CAS PubMed.
- X. H. Gao, Y. Y. Cui, R. M. Levenson, L. W. K. Chung and S. M. Nie, Nat. Biotechnol., 2004, 22, 969–976 CrossRef CAS PubMed.
- N. Nishiyama and K. Kataoka, Pharmacol. Ther., 2006, 112, 630–648 CrossRef CAS PubMed.
- H.-Y. Lian, M. Hu, C.-H. Liu, Y. Yamauchi and K. C. W. Wu, Chem. Commun., 2012, 48, 5151–5153 RSC.
- J. Hu, G. Zhang and S. Liu, Chem. Soc. Rev., 2012, 41, 5933–5949 RSC.
- L. Brannon-Peppas and J. O. Blanchette, Adv. Drug Delivery Rev., 2004, 56, 1649–1659 CrossRef CAS PubMed.
- X. Wang, X. Wang and Z. Guo, Acc. Chem. Res., 2015, 48, 2622–2631 CrossRef CAS PubMed.
- H. Cabral, Y. Matsumoto, K. Mizuno, Q. Chen, M. Murakami, M. Kimura, Y. Terada, M. R. Kano, K. Miyazono, M. Uesaka, N. Nishiyama and K. Kataoka, Nat. Nanotechnol., 2011, 6, 815–823 CrossRef CAS PubMed.
- L. M. Randolph, M.-P. Chien and N. C. Gianneschi, Chem. Sci., 2012, 3, 1363–1380 RSC.
- J. X. Ding, X. L. Zhuang and X. S. Chen, Curr. Pharm. Biotechnol., 2016, 17, 210–211 CAS.
- Y. Shi, Z. Wang, X. Zhang, T. Xu, S. Ji, D. Ding, Z. Yang and L. Wang, Chem. Commun., 2015, 51, 15265–15267 RSC.
- H. Xiong, D. Zhou, Y. Qi, Z. Zhang, Z. Xie, X. Chen, X. Jing, F. Meng and Y. Huang, Biomacromolecules, 2015, 16, 3980–3988 CrossRef CAS PubMed.
- J.-Z. Du, X.-J. Du, C.-Q. Mao and J. Wang, J. Am. Chem. Soc., 2011, 133, 17560–17563 CrossRef CAS PubMed.
- C. E. Callmann, C. V. Barback, M. P. Thompson, D. J. Hall, R. F. Mattrey and N. C. Gianneschi, Adv. Mater., 2015, 27, 4611–4615 CrossRef CAS PubMed.
- H. D. Tang, C. J. Murphy, B. Zhang, Y. Q. Shen, E. A. Van Kirk, W. J. Murdoch and M. Radosz, Biomaterials, 2010, 31, 7139–7149 CrossRef CAS PubMed.
- M. Li, Z. Tang, D. Zhang, H. Sun, H. Liu, Y. Zhang, Y. Zhang and X. Chen, Biomaterials, 2015, 51, 161–172 CrossRef CAS PubMed.
- K. Osada, H. Cabral, Y. Mochida, S. Lee, K. Nagata, T. Matsuura, M. Yamamoto, Y. Anraku, A. Kishimura, N. Nishiyama and K. Kataoka, J. Am. Chem. Soc., 2012, 134, 13172–13175 CrossRef CAS PubMed.
- S. He, D. Zhou, H. Kuang, Y. Wu, X. Jing and Y. Huang, J. Controlled Release, 2015, 213, E96 CrossRef PubMed.
- X. Liu, J. Xiang, D. Zhu, L. Jiang, Z. Zhou, J. Tang, X. Liu, Y. Huang and Y. Shen, Adv. Mater., 2016, 28, 1743–1752 CrossRef CAS PubMed.
- B. P. Bastakoti, K. C. W. Wu, M. Inoue, S.-i. Yusa, K. Nakashima and Y. Yamauchi, Chem.–Eur. J., 2013, 19, 4812–4817 CrossRef CAS PubMed.
- J. L.-L. Tsai, T. Zou, J. Liu, T. Chen, A. O.-Y. Chan, C. Yang, C.-N. Lok and C.-M. Che, Chem. Sci., 2015, 6, 3823–3830 RSC.
- C.-N. Lok, T. Zou, J.-J. Zhang, I. W.-S. Lin and C.-M. Che, Adv. Mater., 2014, 26, 5550–5557 CrossRef CAS PubMed.
- L. A. Ba, M. Doring, V. Jamier and C. Jacob, Org. Biomol. Chem., 2010, 8, 4203–4216 CAS.
- L. J. Edgar, R. N. Vellanki, A. Halupa, D. Hedley, B. G. Wouters and M. Nitz, Angew. Chem., Int. Ed., 2014, 53, 11473–11477 CrossRef CAS PubMed.
- L. Wang, W. Cao and H. Xu, ChemNanoMat, 2016, 2, 479–488 CrossRef CAS.
- D. S. Avila, A. Benedetto, C. Au, F. Manarin, K. Erikson, F. A. Soares, J. B. T. Rocha and M. Aschner, Free Radical Biol. Med., 2012, 52, 1903–1910 CrossRef CAS PubMed.
- J. Thomas, Z. Dong, W. Dehaen and M. Smet, Macromol. Rapid Commun., 2012, 33, 2127–2132 CrossRef CAS PubMed.
- Y. Kalechman, U. Gafter, R. Gal, G. Rushkin, D. H. Yan, M. Albeck and B. Sredni, J. Immunol., 2002, 169, 384–392 CrossRef CAS.
- H. Xu, W. Cao and X. Zhang, Acc. Chem. Res., 2013, 46, 1647–1658 CrossRef CAS PubMed.
- X. Miao, W. Cao, W. Zheng, J. Wang, X. Zhang, J. Gao, C. Yang, D. Kong, H. Xu, L. Wang and Z. Yang, Angew. Chem., Int. Ed., 2013, 52, 7781–7785 CrossRef CAS PubMed.
- W. Cao, Y. Li, Y. Yi, S. Ji, L. Zeng, Z. Sun and H. Xu, Chem. Sci., 2012, 3, 3403–3408 RSC.
- W. Cao, X. Zhang, X. Miao, Z. Yang and H. Xu, Angew. Chem., Int. Ed., 2013, 52, 6233–6237 CrossRef CAS PubMed.
- S. Ji, W. Cao, Y. Yu and H. Xu, Angew. Chem., Int. Ed., 2014, 53, 6781–6785 CrossRef CAS PubMed.
- W. Cao, L. Wang and H. Xu, Nano Today, 2015, 10, 717–736 CrossRef CAS.
- W. Cao, Y. Gu, T. Li and H. Xu, Chem. Commun., 2015, 51, 7069–7071 RSC.
- Q. Xu, C. He, C. Xiao and X. Chen, Macromol. Biosci., 2016, 16, 635–646 CrossRef CAS PubMed.
- W. Cao, Y. Gu, M. Meineck, T. Li and H. Xu, J. Am. Chem. Soc., 2014, 136, 5132–5137 CrossRef CAS PubMed.
- W. Cao, L. Wang and H. Xu, Chem. Commun., 2015, 51, 5520–5522 RSC.
- L. Zeng, Y. Li, T. Li, W. Cao, Y. Yi, W. Geng, Z. Sun and H. Xu, Chem.–Asian J., 2014, 9, 2295–2302 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthetic routes, in vitro and in vivo experiments. See DOI: 10.1039/c6ra19768j |
| This journal is © The Royal Society of Chemistry 2016 |




