Evaluation of the inhibitory potential of HPMC, PVP and HPC polymers on nucleation and crystal growth†
Rahul B. Chavana,
Rajesh Thipparaboinaa,
Dinesh Kumarab and
Nalini R. Shastri*a
aSolid State Pharmaceutical Research Group (SSPRG), Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Balanagar, Hyderabad, 500037, India. E-mail: nalini@niperhyd.ac.in; svcphod@yahoo.co.in; Fax: +91-040-2307375; Tel: +91-040-23423749
bSolid State Pharmaceutical Cluster (SSPC), School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin, The University of Dublin, Ireland
First published on 12th August 2016
Abstract
Supersaturated drug delivery systems are generally developed for improving solubility and dissolution of poorly soluble drugs. Supersaturation is a high energy state with a tendency to precipitate. Polymers are often used as precipitation inhibitors that may act by inhibiting either nucleation or crystal growth, which are important steps of the crystallization process. Hence, proper understanding of the inhibitory potential of polymers on these critical processes is crucial for selection of appropriate polymers for development of supersaturated drug delivery systems. The current study aims to evaluate the inhibitory potential of polymers like HPMC, PVP and HPC on nucleation and crystal growth using a model drug nifedipine. Inhibition of nucleation and crystal growth was studied by measuring the induction time and precipitation initiation time, respectively. In addition, the extent of maintaining supersaturation was determined by defining a new term, the supersaturation holding capacity (SHC) of the polymers. Precipitates obtained were characterized by FTIR and DSC. The contribution of polymer properties on precipitation inhibition was analyzed by partial least square analysis. In the absence of polymer, nifedipine precipitates in 5 min. High viscosity grades of HPMC effectively inhibited nucleation which was reflected by 2–10 fold enhancements in induction time. In addition, they also inhibited the crystal growth which was reflected through 3–4 fold enhancements in SHC. PVP and HPC polymer grades were found to be less effective in nucleation inhibition. Experimental evaluation in addition to partial least square regression analysis highlights the importance of polymer properties (hydrogen bond acceptors, hydroxypropoxy content, methoxy content and viscosity) in precipitation inhibition which could aid in screening and selection of suitable polymers for systematic development of supersaturated drug delivery systems.
Introduction
Supersaturation is a state wherein the concentration of a solute in solution is higher than the equilibrium solubility. It has higher thermodynamic activity which promotes absorption. Supersaturation based drug delivery has received considerable attention as it enhances solubility of poorly soluble molecules.1–3 Among all the supersaturated systems, amorphous solid dispersion is the most extensively studied system with several products in the market. A major hurdle in the development of such supersaturated drug delivery systems is maintenance of the high energy supersaturated state for a sufficient duration and prevention of precipitation. As a result, the benefit of enhanced solubility by using supersaturated drug delivery system may be compromised due to unintended precipitation.1,4,5 Nucleation and crystal growth are two steps involved in precipitation or crystallization. Supersaturation can be maintained if any one of them is inhibited.6–8 For maintenance of supersaturation or prevention of precipitation, precipitation inhibitors are generally used. Polymers used in the development supersaturated drug delivery system, prevent precipitation in solution and crystallization of amorphous drug in solid state.6,7,9 These additives can delay crystallization from supersaturated system by inhibiting either nucleation or crystal growth. Molecular mobility, recrystallization tendency of drug molecule and drug–polymer interactions play a significant role in inhibition of precipitation from supersaturated system, both in solid and solution state.3,10 Numerous research publications in this area have reported the impact of polymers on crystallization inhibition and different mechanisms have been proposed regarding their role.10,11 Few authors have documented the use of precipitation rate and precipitation initiation time (PIT) for calculating inhibitory potential of polymers on crystal growth.10,12 Nucleation is an important step of solution crystallization as it decides the final crystal form and crystal properties. Hence, exploring the impact of polymers on nucleation would be crucial for developing supersaturated drug delivery system with optimal supersaturation states for enhanced absorption.8,13,14 Study of impact of polymers on nucleation is a difficult task as it is a molecular level process and inaccessible to direct experimentation.14 As a result, inhibitory activity of polymers on nucleation kinetics has been indirectly determined by measuring nucleation induction time (IT).8 Although, limited information is available on studies related to inhibitory potential of polymers on crystal growth or nucleation separately, no comparative assessment of inhibitory potential of polymers on both step of crystallization in solution state has been reported to the best of our knowledge. Similarly, it is imperative to understand the effect of polymer properties on nucleation and crystal growth inhibition as it can help in screening of polymers for development of supersaturated drug delivery system as many supersaturated systems have failed due to instability induced by crystallization phenomena.The primary objective of this work was to screen different polymers for maintenance of supersaturation of a poorly soluble drug. Impact of these polymers on nucleation and crystal growth was determined quantitatively using IT and PIT respectively. In addition to IT and PIT, a new parameter, supersaturation holding capacity (SHC) was calculated from the precipitation curve, to measure the duration up to which the polymer can hold supersaturation. This parameter was found useful to obtain a rank order correlation between various polymers and thus aid in screening and selection of polymers for supersaturated drug delivery system. Partial least square analysis (PLS) was also carried out to study the impact of polymer properties on inhibitory effects of polymer on nucleation and crystal growth.
Nifedipine, a 1,4-dihydropyridine calcium channel blocker, was used as a model drug for this study. Nifedipine belongs to BCS class II and is poorly soluble drug with low bioavailability. It belongs to class II of crystallization tendency classification and shows medium crystallization tendency.15 Low aqueous solubility of nifedipine is often found to be responsible for its poor and irregular bioavailability after oral administration.16 Numerous reports on preparation and characterization of solid dispersion of nifedipine are available.17–19 However, most of the dispersions failed to address the issues of physical and chemical stability of nifedipine solid dispersion20,21 since, nifedipine got converted into crystalline state from amorphous form due to elevated temperature and humidity.21 This work attempts to provide rationale for selection of right polymer for development of dispersion of nifedipine with reliable physical and chemical stability based on the potential nucleation and inhibition behaviour of polymers. Physicochemical properties of nifedipine are summarized in Table S1 (ESI†). Various grades of hydroxypropylmethyl cellulose (HPMC), hydroxypropyl cellulose (HPC), and polyvinyl pyrrolidone (PVP) were explored in this study. Molecular weight, viscosity and other properties of polymers are summarized in Table S2.†
Materials and methods
Materials
Nifedipine was received as a generous gift from Mylan Laboratories (Hyderabad, India). HPMC grades (Methocel E3, E5, E6, E15, E50, K4M, K15M and K100M) were obtained as gift samples from Colorcon Asia Private Limited (India). HPC grades (HPC L, HPC M, HPC H, HPC SL and HPC SSL) were kindly gifted by Nippon Soda Co., Ltd., Japan while PVP grades (PVP K17, PVP K25 and PVP K30) were obtained as a gift sample from BASF SE, Germany. Methanol used was of high performance liquid chromatography grade. All the other chemicals and solvents were of analytical grade. Double distilled water was generated in house.Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and the supernatant was collected. The concentration of nifedipine in supernatant was measured by a validated UV spectrometric method at a λmax of 340 nm (JascoV-650 spectrophotometer). All experiments were performed in triplicates and in dark condition.
000 rpm for 15 min and the supernatant was collected. The concentration of nifedipine in supernatant was measured by a validated UV spectrometric method at a λmax of 340 nm (JascoV-650 spectrophotometer). All experiments were performed in triplicates and in dark condition.where C is solution concentration of the solute and Ceq. is its equilibrium solubility.
| Polymer | HA | Vi | S | HP | MC | SR | PIT (min) | IT (min) | t10% | t50% | SHC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPMC E3 | 6 | 3.2 | 0.0371 | 8.8 | 29.4 | 2.1 | 40 | 42.33 | 5.785 | 155.76 | 3.11 |
| HPMC E5 | 6 | 5 | 0.1236 | 9.2 | 28.4 | 0.61 | 50 | 9 | 42.13 | 166.58 | 2.58 |
| HPMC E6 | 6 | 6.2 | 0.0857 | 9.1 | 28.7 | 0.73 | 40 | 28.67 | 53.28 | 161.076 | 2.24 |
| HPMC E15 | 6 | 16.6 | 0.0436 | 9.7 | 28.2 | 1.8 | 70 | 20.67 | 41.91 | 168.296 | 2.62 |
| HPMC E50 | 6 | 48 | 0.1022 | 9 | 28.8 | 0.76 | 90 | 12.5 | 76.04 | 264.9 | 3.92 |
| HPMC K4M | 6 | 4927 | 0.025 | 8.2 | 23.3 | 2.45 | 60 | 33 | 18 | 186.49 | 3.5 |
| HPMC K15M | 6 | 7382 | 0.0228 | 8.6 | 23.1 | 2.65 | 70 | 47.67 | 37.51 | 154.165 | 2.42 |
| HPMC K100M | 6 | 113![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384 384 |
0.0513 | 10.5 | 22.8 | 1.21 | 80 | 73 | 31.5 | 159.45 | 2.65 |
| PVP K17 | 2 | 2.5 | 0.0218 | 0 | 0 | 1.6 | 70 | 13.67 | 58.61 | 182.95 | 2.58 |
| PVP K25 | 2 | 4.5 | 0.0138 | 0 | 0 | 4.53 | 60 | 17.5 | 5.45 | 93.33 | 1.82 |
| PVP K30 | 2 | 7 | 0.0189 | 0 | 0 | 3.42 | 50 | 16.5 | 1.86 | 82.28 | 1.67 |
| HPC L | 16 | 9 | 0.0196 | 65.3 | 0 | 3.6 | 30 | 26.5 | 0 | 107.75 | 2.24 |
| HPC M | 16 | 330 | 0.0204 | 64.8 | 0 | 3.43 | 50 | 40.17 | 38.51 | 104.26 | 1.36 |
| HPC H | 16 | 2350 | 0.0175 | 70.1 | 0 | 4.53 | 40 | 13.17 | 6.09 | 80.04 | 1.53 |
| HPC SL | 16 | 5.2 | 0.0152 | 69.7 | 0 | 4.43 | 50 | 27.66 | 3.05 | 76.07 | 1.51 |
| HPC SSL | 16 | 2.4 | 0.016 | 69.4 | 0 | 4.68 | 30 | 24 | 1.34 | 70.59 | 1.45 |
Results and discussion
Solubility parameter determination
Solubility parameter is an indicator of hydrophobicity22 and is found to be important in selection of polymer for the development of supersaturated drug delivery system.8 It characterizes relative hydrophobicity of polymer with drug. Predicted solubility parameter values of nifedipine, HPMC, PVP and HPC, were found to be 21.41, 22.4, 28.31 and 25.99 respectively. According to Ilevbare et al., if ΔSP between polymer and drug is small then the polymer is found to be a good crystal growth inhibitor.24 Based on this hypothesis, HPMC was predicted to be a significant crystal growth inhibitor as its ΔSP value was 0.99 which was much smaller in comparison to ΔSP values of PVP (6.9) and HPC (4.58).Solubility study
Equilibrium solubility of plain nifedipine was found to be less than 5 μg ml−1. The equilibrium solubility of drug can be changed in presence of polymers hence there is a need to determine the solubility of drug in presence of polymers. Results of the solubility study are presented in Fig. 1. It was observed that the solubility of nifedipine increased significantly in presence of polymers. Optimal enhancement in solubility of nifedipine was observed at 50 μg ml−1 concentration of polymers, and hence was selected for performing precipitation and induction time determination experiments. | ||
| Fig. 1 Solubility of nifedipine in presence of different polymers at different concentration (A) HPMC (B) PVP (C) HPC (n = 3). | ||
Determination of induction time
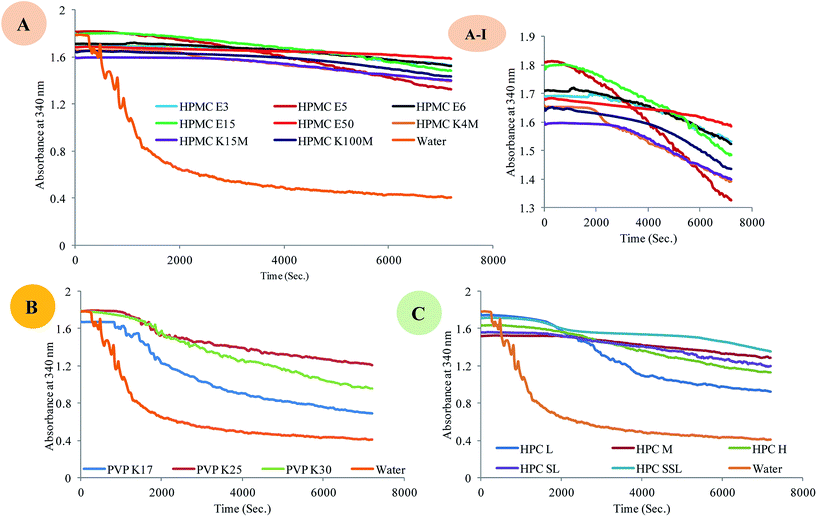
IT is also termed as true nucleation time.6,25 In crystallization kinetics, nucleation time is the time required for the formation of critical nuclei from the supersaturated solution. Detection of crystal nuclei experimentally is not possible until they grow up to detectable size. Hence, experimental IT is the terminology used to describe the time required for formation of observable change in crystallization system from starting point of supersaturation. It is inclusive of true nucleation time tn (time required for formation of critical nucleus) and time required for growth of nuclei to detectable size range (tg).25,26| IT = tn + tg |
IT is not a fundamental property; it mainly depends upon the method used for detection of change in crystallizing media after generation of supersaturation. This emphasizes the importance of method used for determination of IT. Method employed in this study is based on the principle that as nucleation starts and the critical nuclei grows up to a detectable size, a sudden decline in absorbance occurs, which is an indicator of crystallization onset. Rapid decline in absorbance was observed in absence of polymer, indicative of rapid crystallization. As shown in Fig. 2, IT for nifedipine in absence of polymer was less than 5 min. After 10 min, visible macroscopic crystals appeared in solution. Formation of macroscopic crystal leads to formation of turbid system leading to loss in absorbance. Significant enhancement in IT was observed in presence of all polymers. In addition, the absorbance vs. time profile did not show any significant decline at the initial time points in presence of polymers. Extent of inhibition of the IT depends upon specific polymers and their grades. Among all polymers, HPMC K100M showed highest nucleation inhibitory effect. HPMC grades had stronger influence on nucleation as compared to PVP and HPC grades. This was reflected in steady absorbance vs. time profile of HPMC grades when compared to that of PVP and HPC (Fig. 2). High molecular weight and higher viscosity grades of HPMC shows improved nucleation inhibition than lower molecular weight and low viscosity grades possibly due to availability of more number of –OH groups for forming intermolecular interactions with drug. Among various grades of HPMC polymers, the order of enhancement of IT was HPMC K100M > HPMC K15M > HPMC E3 > HPMC K4M > HPMC E6 > HPMC E15 > HPMC E50 > HPMC E5 (Table 1). In case of HPC polymers, maximum nucleation inhibition was observed with HPC M. All HPC grades showed less nucleation inhibition potential when compared to HPMC grades probably due to lower solubility of drug in presence of HPC, less number of –OH groups as compared to HPMC grades and large difference in SP values of HPC and drug (ΔSP = 4.58).
Rank order of HPC polymers for increasing the IT was found to be HPC M > HPC SL > HPC L > HPC SSL > HPC H. Among PVP grades, maximum nucleation inhibition was observed with PVP K25 (Table 1). Rank order of inhibitory potential of PVP grades on nifedipine nucleation was found to be PVP K25 > PVP K30 > PVP K17. Differences in IT values were not large among the PVP grades, which could be due to insignificant difference in their viscosity.
Precipitation study
PIT and precipitation rate are the well known parameters used for studying crystal growth inhibition. Change in concentration was observed after addition of methanolic solution of nifedipine (1 mg ml−1) which was used for determination of PIT. Concentration drop of nifedipine was obtained from the absorbance curve at 340 nm. PIT was identified by determining the first detectable change in the slope of precipitation curve. As shown in Fig. S1,† PIT in the absence of polymer was less than 30 min and t10% and t50% values of nifedipine in absence polymer were 5.8 and 54.01 min respectively (Fig. S2†). These values indicate that the concentration of nifedipine in the precipitation study dropped rapidly during initial time points due to simultaneous nucleation and crystal growth. However, further reduction in concentration occurred at a very slow rate. For 50% reduction in concentration of nifedipine, it took nearly 54 min. Precipitation study parameters PIT, t10% and t50% were increased in the presence of polymers (Fig. S1 and S2†). All polymers extended the PIT of nifedipine. Maximum enhancement in PIT was observed with HPMC (K100M, HPMC E50 and K15M). Rank order of PIT in HPMC grades was found to be HPMC E50 = HPMC K100M > HPMC K15 M = HPMC E15 > HPMC K4M > HPMC E5 > HPMC E6 = HPMC E5. HPC and PVP (except PVP K17) grades increased PIT less effectively. Comparative assessment of precipitation parameters for different polymers revealed that maximum enhancement in t10% were observed with HPMC E50 extending t10% from 5.8 min in absence of polymer to 76.04 min. However, HPC and PVP (except PVP K17) grades were found to be inefficient in enhancing t10% values beyond 5.8 min. Other grades of PVP have shown faster precipitation rate during initial time reflecting low t10% (5.45 and 1.86 min with PVP K25 and PVP K30 respectively). Same trend was also observed with PIT, where HPC and PVP failed to increase PIT significantly. One of the most significant contributing factors for this finding was the hydrophilic nature of both polymers, since it has been reported that hydrophilic polymers may not be effective in precipitation inhibition for hydrophobic drugs.8 Output of the precipitation study highlighted that superior crystal growth inhibitory potential of HPMC polymers could be mainly attributed to closer SP values with the drug (ΔSP = 0.99), higher drug solubility in HPMC and presence of methoxy content which might contribute to intermolecular drug polymer interactions leading to enhanced retention of the drug in the polymer solution system.As shown in Fig. 3, precipitation rate was high at initial time point; however after 30 min it declined and remained constant for some duration. On comparison of the steady phase, it was observed that the concentration of the drug in the supersaturation system at any given time point was significantly higher than the drug solution system in absence of polymer. To determine the duration retaining ability of the supersaturation and their capacity to prevent them from further decline, a new parameter Δt (t50% − t10%) was calculated for the polymers. This parameter which was an indicator of the time required for decline in the concentration from 10% to 50%. In absence of polymer, this value was found to be 48.2 min which increased significantly in presence of all polymers. Maximum enhancement was observed with HPMC E50, which increased Δt (t50% − t10%) to 188.86 min. Similarly, the efficiency of polymer for maintaining supersaturation was also described in terms of a non dimensionless value SHC which would permit for easy quantitative comparison between the polymers for any given supersaturated system. SHC can thus emerge as a promising parameter for polymer selection in this type of drug delivery system, as both IT and PIT values reflect only the initial change in absorbance and do not take into consideration the terminal part of the precipitation curve. It was observed that HPMC possess higher SHC than PVP and HPC which is in accordance to Δt (t50% − t10%) values. HPMC E50 showed SHC near to 3.91 (Table 1). However, other grades of PVP and HPC didn't show much enhancement in SHC except for PVP K17. To summarize, when all parameters for nucleation and crystal growth were considered, HPMC was found to be superior to PVP and HPC due to induction time enhancement with higher viscosity grades and improved SHC with medium viscosity grades of HPMC.
 | ||
| Fig. 3 Concentration versus time curves are derived from de supersaturation assay (A) HPMC grades; (B) PVP grades; (C) HPC grades. | ||
Evaluation of impact of physicochemical properties of polymers on nucleation and crystal growth inhibition
The impact of physicochemical properties of polymers on various nucleation and crystal growth inhibition parameters was studied using PLS analysis. Independent variables related to polymer properties were identified (Table S2†) and preliminary screening for the impact of these independent variables on the dependent variables was determined by obtaining correlation coefficient between them. Five input variables which had correlation coefficients more than 0.5 (solubility, viscosity, hydroxypropoxy content, methoxy content and hydrogen bond acceptor) were identified and retained for PLS analysis (Table 1). On PLS model building, the first two PLS components were able to explain 57.03% variance in Y data (Q2) based on 73.85% variation in X (R2) data. Values of Q2 > 50% is considered acceptable and hence the model was used for identification for influencing factors using a plot of the regression coefficient of polymer properties against response variables (Fig. 4) and correlation loading plot (Fig. 5). | ||
| Fig. 5 Correlation loading plot obtained from PLS analysis. (X) independent (Y) dependent variables. | ||
Variables with high regression coefficient indicate high influence on the responses. The correlation loading plot gives relative importance of the variables. Variables near each other are positively correlated while those opposite to each other from the origin are negatively correlated. Hydroxypropoxy content and hydrogen acceptor were found to be critical factors on inhibition by virtue of their ability to undergo intermolecular interactions, which in turn determined the efficacy of polymers as nucleation inhibitor or crystal growth inhibitors. Viscosity contributed more towards nucleation inhibition than crystal growth inhibition. Contribution of viscosity towards IT was positively correlated (Fig. 5) wherein both variables were close to each other. Along with viscosity, methoxy content also contributed on nucleation inhibition. However, contribution of solubility, hydrogen bond acceptor and hydroxypropoxy content towards nucleation inhibition was poor. Methoxy content and hydroxypropoxy content had negative correlation with each other (Fig. 5). Methoxy content had positive impact on t10%, t50% and SHC which was also reflected in our experimental outcome wherein HPMC due high methoxy content demonstrated superior precipitation inhibition. SHC was dependent on all factors except viscosity (Fig. 4).
Solid state characterization of precipitate
Precipitates of nifedipine in presence and absence of polymers were analyzed by DSC and FTIR to rule out any precipitation induced polymorphism or pseudo polymorphism. Nifedipine possess characteristic IR absorption band at 3331 cm−1 for –OH and amine, 2952 cm−1 for –OH stretching, 1667 cm−1 and 1436 cm−1 corresponding to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and aromatic N–O stretching respectively (Fig. S3†). All FT-IR spectra of precipitates were super imposable with nifedipine spectra, demonstrating that the precipitates of nifedipine were similar in their structural arrangement and conformations. The DSC thermograms of all precipitates were similar to that of plain nifedipine (Fig. S4†). The DSC curve of plain nifedipine showed a single sharp endotherm at 173 °C corresponding to the reported melting point of nifedipine.27 Similarly, precipitates also showed a single melting point endotherm in the range of 168–173 °C. These results ruled out the possibility of polymorphic transition during precipitation of nifedipine in presence of polymer. Possibility of formation of solvates/hydrates was also ruled out as no solvent endothermic peaks were detected in thermograms.
O and aromatic N–O stretching respectively (Fig. S3†). All FT-IR spectra of precipitates were super imposable with nifedipine spectra, demonstrating that the precipitates of nifedipine were similar in their structural arrangement and conformations. The DSC thermograms of all precipitates were similar to that of plain nifedipine (Fig. S4†). The DSC curve of plain nifedipine showed a single sharp endotherm at 173 °C corresponding to the reported melting point of nifedipine.27 Similarly, precipitates also showed a single melting point endotherm in the range of 168–173 °C. These results ruled out the possibility of polymorphic transition during precipitation of nifedipine in presence of polymer. Possibility of formation of solvates/hydrates was also ruled out as no solvent endothermic peaks were detected in thermograms.
Conclusions
In this study, inhibitory potential of polymers on nucleation and crystal growth during precipitation from supersaturated solution was evaluated on a drug with medium crystallization tendency. For a model drug nifedipine, HPMC was found to be an effective polymer; as it inhibits both nucleation and crystal growth, exhibiting highest SHC. A lower value of ΔSP between drug and polymer indicated superior crystal growth inhibition potential of HPMC especially for hydrophobic drug like nifedipine. Higher viscosity grades of HPMC inhibited both steps of crystallization. PVP and HPC were found to be less effective than HPMC in precipitation inhibition of nifedipine from supersaturated solution. Lower viscosity grade of PVP was a good crystal growth inhibitor while higher viscosity grade PVP K30 a good nucleation inhibitor. PLS analysis indicated that precipitation inhibition was mainly governed by polymer properties like viscosity, methoxy content, hydroxypropoxy content, hydrogen bond acceptor and solubility of drug in presence of polymers. However, no significant correlation was observed between monomer number, degree of polymerization, molecular weight, Tg and response variables. This study underlines the importance of polymer properties on nucleation and crystal growth inhibition. Similarly, this study also emphasizes the significance of polymer screening as nucleation and crystal growth inhibitors as both these steps may affect precipitation kinetics.Acknowledgements
The authors acknowledge the financial support from the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India.References
- J. Brouwers, M. E. Brewster and P. Augustijns, J. Pharm. Sci., 2009, 98, 2549–2572 CrossRef CAS PubMed.
- S. Xu and W.-G. Dai, Int. J. Pharm., 2013, 453, 36–43 CrossRef CAS PubMed.
- D. B. Warren, H. Benameur, C. J. Porter and C. W. Pouton, J. Drug Targeting, 2010, 18, 704–731 CrossRef CAS PubMed.
- R. Vandecruys, J. Peeters, G. Verreck and M. E. Brewster, Int. J. Pharm., 2007, 342, 168–175 CrossRef CAS PubMed.
- D. E. Alonzo, G. G. Zhang, D. Zhou, Y. Gao and L. S. Taylor, Pharm. Res., 2010, 27, 608–618 CrossRef CAS PubMed.
- S. Raghavan, A. Trividic, A. Davis and J. Hadgraft, Int. J. Pharm., 2001, 212, 213–221 CrossRef CAS PubMed.
- A. Simonelli, S. Mehta and W. Higuchi, J. Pharm. Sci., 1970, 59, 633–638 CrossRef CAS PubMed.
- G. A. Ilevbare, H. Liu, K. J. Edgar and L. S. Taylor, Cryst. Growth Des., 2012, 13, 740–751 Search PubMed.
- G. A. Ilevbare, H. Liu, K. J. Edgar and L. S. Taylor, CrystEngComm, 2012, 14, 6503–6514 RSC.
- H. Chauhan, A. Kuldipkumar, T. Barder, A. Medek, C.-H. Gu and E. Atef, Pharm. Res., 2014, 31, 500–515 CrossRef CAS PubMed.
- N. S. Trasi and L. S. Taylor, CrystEngComm, 2012, 14, 5188–5197 RSC.
- H. Chauhan, C. Hui-Gu and E. Atef, J. Pharm. Sci., 2013, 102, 1924–1935 CrossRef CAS PubMed.
- N. Rodríguez-hornedo and D. Murphy, J. Pharm. Sci., 1999, 88, 651–660 CrossRef PubMed.
- J. Anwar, P. K. Boateng, R. Tamaki and S. Odedra, Angew. Chem., Int. Ed., 2009, 48, 1596–1600 CrossRef CAS PubMed.
- S. A. Raina, B. V. Eerdenbrugh, D. E. Alonzo, H. Mo, G. G. Zhang, Y. Gao and L. S. Taylor, J. Pharm. Sci., 2015, 104, 1981–1992 CrossRef CAS PubMed.
- B. Devarakonda, R. A. Hill and M. M. de Villiers, Int. J. Pharm., 2004, 284, 133–140 CrossRef CAS PubMed.
- C.-W. Lin and T.-M. Cham, Int. J. Pharm., 1996, 127, 261–272 CrossRef CAS.
- I. Sugimoto, A. Kuchiki, H. Nakagawa, K. Tohgo, S. Kondo, I. Iwane and K. Takahashi, Drug Dev. Ind. Pharm., 2008, 6, 137–160 CrossRef.
- S. R. Vippagunta, K. A. Maul, S. Tallavajhala and D. J. Grant, Int. J. Pharm., 2002, 236, 111–123 CrossRef CAS PubMed.
- T. Save and P. Venkitachalam, Drug Dev. Ind. Pharm., 1992, 18, 1663–1679 CrossRef CAS.
- H. Suzuki and H. Sunada, Chem. Pharm. Bull., 1998, 46, 1015–1020 CrossRef CAS.
- R. F. Fedors, Polym. Eng. Sci., 1974, 14, 147–154 CAS.
- D. Prasad, H. Chauhan and E. Atef, Mol. Pharm., 2016, 13, 756–765 CrossRef CAS PubMed.
- G. A. Ilevbare, H. Liu, K. J. Edgar and L. S. Taylor, Cryst. Growth Des., 2012, 12, 3133–3143 CAS.
- A. Kuldipkumar, G. S. Kwon and G. G. Zhang, Cryst. Growth Des., 2007, 7, 234–242 CAS.
- O. Söhnel and J. W. Mullin, J. Colloid Interface Sci., 1988, 123, 43–50 CrossRef.
- D. Grooff, M. De Villiers and W. Liebenberg, Thermochim. Acta, 2007, 454, 33–42 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1 physicochemical properties of nifedipine, Table S2 properties of polymers, Table S3 stability assessment by UV-visible method, Fig. S1 PIT and IT of nifedipine in presence and absence of polymers, Fig. S2 t10%, and t50% parameters obtained after precipitation of nifedipine in absence and presence of polymer, Fig. S3 FT-IR spectra of precipitates of nifedipine in absence and presence of polymers and Fig. S4 DSC thermograms of precipitates of nifedipine (NIF) in absence and presence of polymers with plain drug. See DOI: 10.1039/c6ra19746a |
| This journal is © The Royal Society of Chemistry 2016 |