Insertion behavior of imidazolium and pyrrolidinium based ionic liquids into α and β-cyclodextrins: mechanism and factors leading to host–guest inclusion complexes†
Abstract
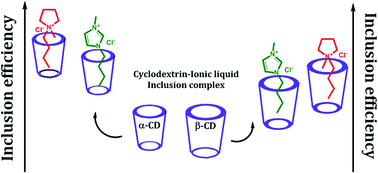
Host–guest inclusion complexes formed from two ionic liquids (namely, 1-butyl-3-methylimidazolium chloride and 1-butyl-1-methylpyrrolidinium chloride) with α and β-cyclodextrin have been investigated by physicochemical and spectroscopic methods as stabilizers, carriers and regulatory releasers of the guest molecules. 1H NMR, 2D ROESY NMR, FT-IR and ESI MS studies confirm the inclusion phenomenon, whereas surface tension and conductivity studies indicate a 1 : 1 stoichiometry of the inclusion complexes. The interactions of cyclodextrin with the two named ionic liquids were characterized by density, viscosity and refractive index measurements, while the binding constants have been evaluated using a non-linear programme by the conductivity method, indicating a higher degree of encapsulation in the case of α-cyclodextrin than that in β-cyclodextrin. The formation of the inclusion complexes was elucidated by hydrophobic effects, structural effects, electrostatic forces and H-bonding interactions.

 Please wait while we load your content...
Please wait while we load your content...