Anaerobic vs. aerobic preparation of silicon nanoparticles by stirred media milling. The effects of dioxygen, milling solvent, and milling time on particle size, surface area, crystallinity, surface/near-surface composition, and reactivity†
Abstract
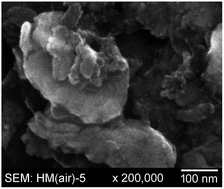
A stirred media mill was hermetically sealed in a high-purity dinitrogen atmosphere glovebox and used to prepare silicon nanoparticles (SiNPs) by milling a slurry of 45–90 μm pieces of metallurgical-grade silicon (MGS) suspended in anhydrous heptane (HEP), heptane containing a small amount of mesitylene (HEP/MES), or mesitylene containing 22 mM pyrene (MES/PYR) under anaerobic and anhydrous conditions (≤5 ppm dioxygen (O2) and water (H2O) vapor). Excess solvent and other volatiles were removed using a rotary evaporator also housed in the glovebox. The SiNPs were further dried at 22 ± 1 °C under vacuum (≤1 × 10−5 Torr) for at least 15 h. The dark-brown or black pyrophoric nanoparticle powders were stored in a high-purity argon atmosphere glovebox. Samples were removed from the glovebox and analyzed by BET surface area (BET-SA) determination, tensimetric titrations with various pressures of O2, PXRD, SEM, TEM, ATR-FTIR, and/or XPS. The effects of (i) anaerobic vs. aerobic milling, (ii) milling fluid, and (iii) milling time on BET-SA, degree of crystallinity, reactivity with O2, and FTIR- or XPS-determined surface/near-surface composition of the SiNPs are discussed. The BET-SA of SiNPs milled anaerobically for 5 h in MES/PYR (258 m2 g−1) was ca. twice as large as the BET-SA for SiNPs milled aerobically in the same milling fluid. In contrast, the BET-SA of SiNPs milled anaerobically in HEP (40 m2 g−1) was four times smaller than for SiNPs milled aerobically in HEP. The PXRD-determined degree of crystallinity steadily decreased and the BET-SA steadily increased over time (1, 2, or 5 h) for SiNPs milled anaerobically in MES/PYR. The degree of crystallinity of SiNPs milled anaerobically in HEP also steadily decreased over time, but the BET-SAs for 1, 2, 3, 4, and 5 h milled samples were essentially the same. MES/PYR milled SiNPs reacted more slowly with O2 and irreversibly absorbed less O2 than HEP milled SiNPs.


 Please wait while we load your content...
Please wait while we load your content...