Synthesis of dense arrays of multiferroic CoFe2O4–PbZr0.52Ti0.48O3 core/shell nanocables
Abstract
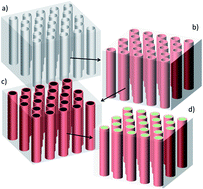
A major challenge in the development of efficient magnetoelectric nanocomposites is the adequate control of the interfaces, in order to avoid the formation of undesirable interphases and to ensure an optimal strain mediated coupling. In this work we used a combination of low-cost impregnation and electrodeposition processes within the ordered nanochannels of a porous anodic alumina template, to elaborate CoFe2O4–PbZr0.52Ti0.48O3 core/shell nanocable arrays with high interfacial areas between the ferroelectric and the magnetic phases. We have shown in this way that the thermal annealing steps required for phase's crystallization and oxidation of metallic CoFe2 into spinel CoFe2O4 are critical with respect to interdiffusion phenomena through the interfaces. The impact of the processing temperature on the morphological and structural features of the nanocables was then discussed. We demonstrated that an optimization of process variables in the synthesis of CoFe2O4–PbZr0.52Ti0.48O3 nanocables allows a significant improvement in their microstructure and ensures the chemical integrity of the two-phase materials, an important concern when seeking for enhanced multiferroic properties.

 Please wait while we load your content...
Please wait while we load your content...