DOI:
10.1039/C6RA19443E
(Paper)
RSC Adv., 2016,
6, 102453-102461
Enhanced drug release by selective cleavage of cross-links in a double-cross-linked hydrogel†
Received
1st August 2016
, Accepted 13th October 2016
First published on 13th October 2016
Abstract
In the present paper, we report on the synthesis and characterization of redox sensitive double-cross-linked poly(acrylic acid) hydrogels using two different cross-linking agents, Jeffamine® and cystamine. The amount of two cross-linking agents was varied in order to synthesize hydrogels with different mechanical strengths. Jeffamine provides mechanical stability to the hydrogels while cystamine incorporates redox sensitivity. The stress values at the break point of the mono- and double-cross-linked hydrogels were determined from stress–strain plots. The disulphide bonds (S–S) in the cystamine were cleaved selectively in the presence of dithiothreitol, which increased the degree of hydrogel swelling. This phenomenon of in situ breaking of one cross-linking and increasing the swelling ratio could be used in swelling-controlled drug delivery systems. The implication of selective breaking of cross-links on the swelling-controlled release of the anticancer drug doxorubicin was demonstrated. We also successfully prepared Ag nanoparticles in the dual cross-linked hydrogels in order to incorporate antibacterial properties and studied their release by selective cleavage of cystamine bonds. These double-cross-linked hydrogels show great promise in drug delivery and tissue engineering applications.
1. Introduction
The controlled release of active ingredients such as drugs, proteins and other micronutrients at the required site on demand is a major challenge. In this context, smart responsive polymeric hydrogels are promising materials for drug delivery1,2 and tissue engineering applications3–5 due to their controlled swelling–deswelling kinetics and tunable properties. Hydrogels contain hydrophilic polymeric chains that are cross-linked either physically6 or chemically,7 thus imparting a 3D network structure to the hydrogel. Major efforts are continuously underway in designing and synthesizing smart hydrogels that are sensitive to external stimuli such as temperature,8,9 pH,1,9–13 and magnetic14 and electric fields.15 Recently, redox stimuli have become important in hydrogels,4,16–18 as volume transition occurs by breaking and reformation of cross-links triggered by reduction and oxidation. Further, the redox stimuli provide rapid and reversible switching between the oxidized and reduced states.
Zrinyi et al.19 reported on the redox-responsive, biocompatible hydrogels based on poly(aspartic acid) (PASP). They synthesized PASP hydrogels simultaneously using two different cross-linking agents, cystamine and diaminobutane. The advantages of PASP hydrogels in terms of their biodegradability, biocompatibility, and non-toxicity are emphasized. The cystamine cross-linking agent which contains disulphide (S–S) bonds could be cleaved using reducing agents such as glutathione and dithiothreitol (DTT). Consequently, the degree of cross-linking of the hydrogel was reduced, which resulted in increasing the swelling ratio of the hydrogel. However, the hydrogels based on PASP exhibited weak mechanical strength and were prepared in toxic solvents such as DMSO and DMF. In addition, gelation times were long, in the order of 2–3 days. Gyarmati et al.20 reported on the reversible response of the PASP hydrogel to external redox and pH stimuli. The hydrogels were prepared with pendant thiol linkages and covalent bonds were formed by in situ cross-linking with diaminobutane. The formation of dual cross-linking was later demonstrated by oxidation resulting in disulphide linkages. However, the implication of this on swelling controlled drug delivery has not yet been reported. Ming Zhong et al.21 reported on dually cross-linked poly(acrylic acid) hydrogels that showed superior mechanical properties with high water absorbancy. The dual cross-linking was effected by covalent linking with bis-acrylamide and reversible dynamic linking by Fe3+ ions. However, the dynamics of breaking and reformation of the ionically linked network under deformation are not clear. Further, the selective breaking of one network and its influence on swelling and controlled drug delivery in these networks has not been reported. This concept of making double-cross-linked hydrogels and breaking of one cross-linking independently is quite interesting and has important technological implications that have not yet been explored.
In the present work, we synthesized double-cross-linked poly(acrylic acid) based hydrogels using two different cross-linking agents, namely Jeffamine (Jeff Huntsman, amine terminated copolymer of PEO–PPO; abbreviations jeffamine and jeff) and cystamine (cys). Jeffamine provides good mechanical strength, while the cystamine with disulphide bonds incorporates redox sensitivity to hydrogels. The hydrogels were easily prepared in aqueous medium at room temperature and the gelation could be completed within a few minutes. Poly(acrylic acid) (PAA)-based hydrogels have been extensively studied for controlled drug delivery applications.1,9,12,22,23 Large numbers of pharmaceutical formulations of FDA-approved creams and ointments contain PAA that acts as a rheological control agent. The double-cross-linked hydrogels based on PAA showed remarkable increase in the mechanical strength as compared to the double-cross-linked PASP hydrogels. With one step ahead, we also demonstrate the selective cleavage of one cross-linking by DTT and its implications on the controlled release of an anticancer drug, doxorubicin (dox) and an antimicrobial Ag nanoparticle.
2. Experimental
2.1 Materials
Poly(acrylic acid) (PAA) (MW = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), cystamine di-hydrochloride, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), dithiothreitol (DTT) were obtained from Sigma-Aldrich, USA. Jeffamine (MW = 2000) was obtained from Texaco Chemical Company, USA. Doxorubicin HCl was obtained from Prolab Marketing Pvt. Ltd., New Delhi. Silver nitrate (AgNO3) was procured from S.D. Fine Chemicals Ltd. (India) and sodium borohydride (NaBH4) was purchased from Merck (India). All chemicals were of analytical grade and used as received.
000), cystamine di-hydrochloride, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), dithiothreitol (DTT) were obtained from Sigma-Aldrich, USA. Jeffamine (MW = 2000) was obtained from Texaco Chemical Company, USA. Doxorubicin HCl was obtained from Prolab Marketing Pvt. Ltd., New Delhi. Silver nitrate (AgNO3) was procured from S.D. Fine Chemicals Ltd. (India) and sodium borohydride (NaBH4) was purchased from Merck (India). All chemicals were of analytical grade and used as received.
2.2 Synthesis of cross-linked PAA hydrogels
300 mg of PAA was dissolved in distilled water and stirred until the solution became homogeneous. To this were added various compositions of cystamine dihydrochloride and jeffamine previously dissolved in small amounts of distilled water. Stirring of the solution continued for about 10 min or until it became homogeneous. The pH of the solution was adjusted to 7.5–8.0 by adding 1 M NaOH solution followed by addition of EDC·HCl. The total volume of the reaction was kept constant at 10 mL for all experiments. After stirring for approximately 10 s, the solution was poured immediately into cylindrical Teflon molds of different sizes. Gelation took place in 10–15 min. Then the gels were removed from molds and kept in distilled water for 4 days with periodic replenishment with fresh water for removal of unreacted reagents and polymer. PAA hydrogels containing both the cross-linking agents in different molar ratios were prepared. The stoichiometry for the synthesis is provided in Table 1. The obtained PAA hydrogels are denoted by PAA-xcys or PA-xjeff where “x” denotes the mol% of the cys/jeff added.
Table 1 Stoichiometry for synthesis of PAA hydrogels
| Sample |
PAA (mol × 10−3) |
Cystamine (mol × 10−3) |
Jeffamine (mol × 10−3) |
EDC·HCl (mol × 10−3) |
| PAA-5cys |
4.17 |
0.208 |
0 |
1.04 |
| PAA-7.5cys |
4.17 |
0.312 |
0 |
1.56 |
| PAA-10cys |
4.17 |
0.417 |
0 |
2.085 |
| PAA-5jeff |
4.17 |
0 |
0.208 |
1.04 |
| PAA-7.5jeff |
4.17 |
0 |
0.312 |
1.56 |
| PAA-10jeff |
4.17 |
0 |
0.417 |
2.085 |
| PAA-2.5cys + 7.5jeff |
4.17 |
0.104 |
0.312 |
2.085 |
| PAA-5cys + 5jeff |
4.17 |
0.208 |
0.208 |
2.085 |
| PAA-7.5cys + 2.5jeff |
4.17 |
0.312 |
0.104 |
2.085 |
2.3 Preparation of porous cross-linked PAA hydrogels
Porous PAA hydrogels were prepared by cryogenic treatment of fully swollen hydrogels followed by removal of ice crystals by lyophilization. The fully swollen PAA hydrogels (12 mm × 12 mm) were placed in liquid nitrogen until complete freezing and then freeze dried in lyophilizer for 24 h to obtain porous hydrogels (see ESI, Fig. 1S†).
2.4 Swelling behavior of hydrogels
The swelling behavior of PAA hydrogels was studied using the gravimetric method. As-prepared hydrogels were immersed in distilled water at room temperature for several days to remove unreacted reactants. The hydrogels were then dried completely in an oven until reaching the constant weight. These xerogels were then immersed in four different solvents—distilled water, PBS (pH 7.4, 0.01 M), sodium citrate–citric acid buffer (pH 3, 0.01 M) and NaCl (0.1 M) kept at 37 °C. The gels were removed and gently pressed between two filter papers to remove any excess solvent on the surface and weighed in an analytical balance. The equilibrium swelling ratio (Qe) was determined using the formula:| |
 | (1) |
where Ws and Wd are the weights of the swollen and dried samples, respectively.
For the kinetics studies, the swelling ratio was measured as a function of time and the time-dependent water uptake was determined using
where
k is the swelling rate constant and
n is the swelling exponent.
2.5 Mechanical properties
The stress–strain measurements in the unidirection were carried out using Instron 5943 mechanical tester at room temperature. The compressive tests were performed on both as-prepared hydrogels and equilibrium swollen hydrogels in PBS (pH 7.4, 0.01 M). The cylindrical test specimens had dimensions of 15 mm diameter × 15 mm height. The compression speed was 2 mm min−1 with a pre-tension load of 1 kN. For each test, three representative samples were obtained and the average value of the results was recorded.
2.6 In situ gelation
The in situ gelation of PAA with two cross-linking agents in aqueous medium was studied using Anton Paar MCR-301 controlled stress rheometer with cup and bob geometry (CC17). PAA aqueous solutions with different molar ratios of cross-linking agents (cystamine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) jeffamine) and EDC coupling agents were placed in a cup and bob. The parameters, storage modulus (G′) and loss modulus (G″) were measured as a function of time at 25 °C. The percentage strain was 0.15% and the frequency was 10 Hz throughout the experiment. The time at which the crossover between G′ and G″ occurs was recorded as the time of gelation.
jeffamine) and EDC coupling agents were placed in a cup and bob. The parameters, storage modulus (G′) and loss modulus (G″) were measured as a function of time at 25 °C. The percentage strain was 0.15% and the frequency was 10 Hz throughout the experiment. The time at which the crossover between G′ and G″ occurs was recorded as the time of gelation.
2.7 Drug loading and release from hydrogel
For the drug loading into the hydrogel, fully dried double-cross-linked PAA gel (xerogel) was immersed in 10 mL of PBS (pH 7.4, 0.01 M) solution containing 0.5 mg of the drug, doxorubicin hydrochloride. The container was kept in a shaking water bath at 37 °C for 36 h. After the gel reached the equilibrium swelling, the swollen gel was removed and oven-dried at 30 °C until reaching the constant weight. The supernatant liquid was collected and assayed by UV-vis spectroscopy at 478 nm. The percentage loading of dox in PAA hydrogel was estimated by the following formula:| |
 | (3) |
where Wx is the amount of dox added in PBS solution, Wy is the amount of dox in supernatant solution after the swelling of the hydrogel and Wt is the total amount of the dox incorporated into the gel.
The release of dox from the PAA xerogels was studied using the following procedure: the dox-loaded PAA xerogels were immersed in 10 mL of PBS solution containing 1 mM DTT in a sample tube kept in a water bath at 37 °C with gentle shaking. The control xerogels were immersed in 10 mL PBS solution without DTT. At predetermined time intervals, a 1 mL aliquot was drawn from the tubes and replenished with same amount of fresh PBS. The amount of dox present in the collected PBS was determined by UV-vis spectrometry. The % dox release was calculated using the following equation:
| |
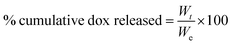 | (4) |
where
Wt is the weight of the dox released at time
t and
We is the total amount of dox loaded.
2.8 Microstructural characterization
Scanning electron microscopy (SEM) was used to investigate the morphology of the hydrogels using the Quanta 200 3D dual beam with electron source of tungsten (W) filament and emission at resolution of 20 kV in high vacuum. The hydrogels were lyophilized and sputter coated with a thin layer of gold. EDAX measurements were carried out to confirm the presence of AgNPs in the hydrogels. Transmission electron microscopy (TEM) was executed on a JEOL (JEM 2000) operating at 200 kV. The sample was prepared by sonicating the AgNPs obtained in the supernatant and dispersing it over a copper grid. The copper grid was dried for 1 day before analysis.
2.9 Silver nanoparticles (AgNPs) loading and release from hydrogel
Preparation of Ag nanoparticles in the hydrogels was carried out using the following method.24 The completely dried PAA-5cys-5jeff hydrogels were immersed in 5 mM solution of silver nitrate overnight to reach equilibrium. The gels were then washed thoroughly to remove silver nitrate from the surface. The swollen gels were then immersed in 10 mM sodium borohydride solution for about 2 h to obtain AgNPs in the gels. The formation of the AgNPs was observed by the change in color of the gels to dark brown. The percentage loading of AgNPs in PAA hydrogel was estimated by completely degrading the hydrogels embedded with AgNPs using the probe sonicator and measuring the UV absorbance of the outside solution. The formation of AgNPs in the hydrogels was confirmed by UV-vis spectroscopy (ESI, Fig. 2S†).
The release of AgNPs from the PAA hydrogels was studied using the following procedure. The AgNP-loaded PAA hydrogels were immersed in 10 mL of distilled water containing 1 mM DTT in a sample tube kept in a water bath at 37 °C with gentle shaking. The control hydrogels were immersed in 10 mL PBS solution without DTT. At predetermined time intervals, a 1 mL aliquot was drawn from the tube and replenished with the same amount of fresh distilled water. The amount of AgNPs present in the collected distilled water was determined by UV-vis spectroscopy at 400 nm. The % AgNPs release was calculated using eqn (4), where Wt is the weight of the AgNPs released at time t and We is the total amount of AgNPs loaded.
3. Results and discussion
3.1 Synthesis of double-cross-linked PAA hydrogels
The double-cross-linked PAA hydrogels were prepared simultaneously using two different cross-linking agents, cystamine and jeffamine. The coupling reaction between the –COOH groups of PAA and the –NH2 groups of cross-linking agents was performed using a water-soluble EDC coupling agent. Unlike the previous report on the synthesis of double-cross-linked poly(aspartic acid) hydrogels in DMSO solvent, which is toxic and undesirable,19 we prepared double-cross-linked PAA hydrogels in an aqueous medium under mild basic conditions (pH = 8). The coupling reaction was found to be faster at this pH. Further, the hydrogels could be synthesized within 3–5 min depending on the amount of cross-linking agents used for the reaction. Hydrogels containing both the cross-linking agents in different mole ratios were prepared; the stoichiometry for the synthesis is shown in Table 1. The reaction pathway for the cross-linking between PAA and the two cross-linking agents is shown in Scheme 1. The advantages of both jeffamine and cystamine are solubility in water and providing a homogeneous aqueous reaction medium for the cross-linking reaction. The obtained hydrogels were completely transparent in the dry and swollen state and exhibited good mechanical strength in the swollen condition. Fig. 1 shows images of dry, as-prepared and fully swollen hydrogels in water.
 |
| | Scheme 1 Reaction schema for synthesis of PAA-cys-jeff double-cross-linked hydrogels. | |
 |
| | Fig. 1 Cross-linked PAA-5jeff hydrogel (a) dried in oven, (b) as-prepared, and (c) fully swollen in distilled water. | |
3.2 Structural characterization
FT-IR spectra of the hydrogels were recorded on a PerkinElmer (USA) device in a diffuse reflectance spectroscopy mode (DRS). The pellets of the dried hydrogels were made using KBr powder. In the FTIR spectra, the characteristic peaks at 1560 cm−1 and 1654 cm−1 appear due to stretching mode of –NH groups and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively. These two peaks in the hydrogels indicate the formation of amide linkages between PAA and cystamine/jeffamine by EDC coupling reaction. On addition of DTT, in PAA-5cys + 5jeff hydrogel, a sharp peak appears at 2874 cm−1. This peak is attributed to the formation of –SH groups by successful breaking of S–S groups selectively in PAA hydrogel in the presence of DTT25 as shown in Fig. 2.
O groups, respectively. These two peaks in the hydrogels indicate the formation of amide linkages between PAA and cystamine/jeffamine by EDC coupling reaction. On addition of DTT, in PAA-5cys + 5jeff hydrogel, a sharp peak appears at 2874 cm−1. This peak is attributed to the formation of –SH groups by successful breaking of S–S groups selectively in PAA hydrogel in the presence of DTT25 as shown in Fig. 2.
 |
| | Fig. 2 FTIR spectra of PAA-5jeff in presence and absence of DTT. | |
3.3 In situ gelation
In the in situ gelation study, PAA, jeffamine, cystamine, NaOH was homogeneously mixed and charged to the cup. Immediately after the addition of EDC coupling agent, G′ and G″ were measured as a function of time. The crossover point of G′ and G″ was recorded as the gelation time. We show in Table 2 the time of gelation (averaged with repetition of three samples) for hydrogels with different molar ratios of jeffamine and cystamine. It is apparent given the results that in all the samples, gelation could take place in 1 min. In particular, the cross-linking agent jeffamine seems to be more reactive than cystamine, wherein the sample with more jeffamine showed faster gelation in 30 s. Similar gelation times were also observed outside the rheometer in molds where gelation can be observed with the naked eye. These remarkably rapid gelation times were comparable to gelation times observed in photo-cross-linking methods.
Table 2 Gelation time for PAA hydrogels with various cross-linking agents
| Sample |
Gelation time (s) |
| PAA-2.5cys + 7.5jeff |
30 |
| PAA-5cys + 5jeff |
45 |
| PAA-7.5cys + 2.5jeff |
60 |
3.4 Swelling behavior
First, we show in Fig. 3 the comparative study of equilibrium swelling ratios of mono-cross-linked PAA hydrogels using jeffamine and cystamine independently. The mol% of cross-linking agent was 5.0, 7.5 and 10.0. The equilibrium swelling ratios were measured in water, 0.1 M NaCl and the two buffer solutions, PBS (pH 7.4, 0.01 M), sodium citrate–citric acid buffer (pH 3, 0.01 M). It is apparent that all hydrogels showed very high swelling in water but exhibited low swelling in NaCl and buffer solutions. This is indeed expected. The hydrogels being polyelectrolyte in nature, the polymer chain dimensions are strongly affected by the presence of salt and reduce the electrostatic repulsion in the expanded polymer chain resulting in contraction of the chains. This in turn reduces the equilibrium swelling of hydrogel. Similarly, in the case of buffer solutions the cations effectively screen the charge repulsion and reduce the degree of swelling. It is well known that the swelling in hydrogels strongly depends on the degree of cross-linking. The swelling decreases as the degree of cross-linking increases. This is clearly observed in equilibrium swelling ratios of all the hydrogels in water, NaCl and buffer solutions. However, it is important to note here that the equilibrium swelling ratios of cystamine cross-linked hydrogels were found to be higher than the equilibrium swelling ratios of jeffamine cross-linked hydrogels at the same mol% of the cross-linkers. This is contrary to the fact that cystamine is a small molecule and can undergo more cross-linking with tight network structure and expected to show lower equilibrium swelling ratios. The higher swelling ratios in this case can be explained on the basis that disulphide bonds in cystamine are susceptible to basic environment and can be cleaved at the reaction pH of 8.0. Therefore, the incorporation of cystamine molecules which form the effective cross-links in the network structure could be less and as a result show higher equilibrium swelling ratios.
 |
| | Fig. 3 Equilibrium swelling ratios of mono-cross-linked PAA hydrogels in water, NaCl (0.1 M), PBS (pH 7.4, 0.01 M), sodium citrate–citric acid buffer (pH 3, 0.01 M). | |
3.5 Swelling behavior of double-cross-linked hydrogels
Fig. 4 presents the equilibrium swelling ratios of double-cross-linked hydrogels in different solvents. The kinetics of swelling of double-cross-linked hydrogels in three different solvents – water, PBS (pH 7.4, 0.01 M), NaCl (0.1 M) was also studied using xerogels of known weight with precise dimensions (cylinders of 12 mm height × 12 mm diameter). The swelling ratios of hydrogels were measured with respect to time until they reached the equilibrium swelling, which took about ∼25 h. The plots of swelling ratios versus time were constructed (see ESI, Fig. 3S†). The swelling exponent n was calculated from the slope of the linear region in the initial swelling profile. The value of swelling exponent n was found to be 0.5–1.0, indicating the non-Fickian behavior of the swelling.
 |
| | Fig. 4 Equilibrium swelling ratios of double-cross-linked PAA hydrogels in water, NaCl (0.1 M), PBS (pH 7.4, 0.01 M). | |
3.6 Selective breaking of one cross-linking in double-cross-linked hydrogel and influence on swelling
The synthesized double-cross-linked PAA hydrogels contained both cystamine and jeffamine cross-linking agents. The disulphide linkage (S–S) in cystamine can be cleaved by redox reagents such as dithiothreitol (DTT) due to significant difference in their redox potentials. Accordingly, the equilibrium swollen double-cross-linked PAA hydrogels were immersed in water, NaCl and PBS solutions containing DTT. The schematics of selective breaking of S–S bonds in cystamine without affecting the jeffamine cross-links is shown in Scheme 2. The selective breaking of S–S bonds in cystamine resulting in decrease in the degree of cross-linking was manifested in the increase in equilibrium swelling ratios of hydrogels. Fig. 5 shows the equilibrium swelling ratios of PAA hydrogels in water, NaCl and PBS (pH 7.4, 0.01 M) solution after the cleavage of cystamine cross-linking. It can be seen that there is a significant increase in the swelling ratio due to decrease in the degree of cross-linking as a result of the breaking of S–S bonds in cystamine. By judiciously varying DTT concentration, the degree of swelling in hydrogels can be controlled, which has major implications in drug delivery applications. These aspects are discussed in subsequent sections.
 |
| | Scheme 2 Selective cleavage of cystamine in presence of DTT in double-cross-linked PAA hydrogels. | |
 |
| | Fig. 5 Equilibrium swelling ratios of double-cross-linked PAA hydrogels in water, NaCl (0.1 M), PBS (pH 7.4, 0.01 M) in presence of DTT. | |
3.7 Mechanical properties
The stress values at break/rupture of the as-prepared and equilibrium swollen mono- and double-cross-linked PAA hydrogels were measured from the stress vs. strain curves using Instron 5943 in the compression mode (ESI, Fig. 4S†). Results shown in Table 3 reveal that in both the mono-cross-linked hydrogels independently by cystamine and jeffamine, the ultimate stress values (i.e. the stress values at break) increase with the increase in degree of cross-linking indicating the enhancement in the mechanical strength of the hydrogels. Interestingly, jeffamine cross-linked hydrogels showed higher ultimate stress values (6–8 times) as compared to cystamine cross-linked hydrogels. This is also reflected in the double-cross-linked PAA hydrogels where the jeffamine content increased systematically. This clearly indicates that jeffamine undergoes more efficient cross-linking in the hydrogel formation. It is also observed that the equilibrium swollen hydrogels showed lower ultimate stress values and lower mechanical strength as compared to the as-prepared hydrogels. This could be attributed to the fact that equilibrium swollen hydrogels contain large amount of fluid, which makes them soft, resulting in lower mechanical strengths. We also show in Fig. 6(b) and (c) the incremental Young's modulus of the mono- (jeffamine cross-linked) and double-cross-linked PAA hydrogels with respect to strain%. It is seen that the modulus increases with the degree of cross-linking in the hydrogels, which is in agreement with the earlier report.26
Table 3 Stress at break point of mono- and double-cross-linked PAA hydrogels with different degree of cross-linking
| Hydrogel sample |
Stress at break point (kPa) |
| As prepared |
Equilibrium swollen in PBS |
| PAA-5cys |
10 |
6.0 |
| PAA-7.5cys |
11 |
8.0 |
| PAA-10cys |
17.1 |
12.5 |
| PAA-5jeff |
51.4 |
33.5 |
| PAA-7.5jeff |
72.6 |
65.4 |
| PAA-10jeff |
130.5 |
125 |
| PAA-7.5cys + 2.5jeff |
47.2 |
35.3 |
| PAA-5cys + 5jeff |
82.7 |
69.2 |
| PAA-2.5cys + 7.5jeff |
103.2 |
91.2 |
 |
| | Fig. 6 (a) Image of an Instron 5943 during compression study of as-prepared PAA-5jeff hydrogel. Incremental Young's modulus with respect to strain% of (b) mono- (jeffamine) and (c) double-cross-linked PAA hydrogels. | |
3.8 Drug loading and release from double-cross-linked PAA hydrogels
In order to determine implications of selective breaking of one cross-linking in the drug delivery applications, we incorporated an anticancer drug, doxorubicin hydrochloride, into double-cross-linked PAA hydrogel (PAA-5cys + 5jeff) and studied the release of dox in PBS (pH 7.4, 0.01 M) upon selective breaking of disulphide (S–S) bonds in cystamine using DTT. The loading of dox into the hydrogel was performed by immersing the dry PAA-5cys + 5jeff xerogel into PBS containing dox hydrochloride. The swelling of the hydrogel induced the diffusion of dox into the hydrogel. Fig. 7 displays results of the cumulative release of dox from PAA-5cys + 5jeff as a function of time with and without the treatment of DTT. As seen in the figure, the hydrogel without the treatment of DTT showed ∼15.0% dox release in 40–80 h, whereas the DTT treated hydrogel cleaved the disulphide linkage and reduced the degree of cross-linking and in turn increased the swelling of hydrogel. The increase in swelling of the hydrogel influenced the faster and higher amount (∼60%) of dox release from the hydrogel over a period of 40–80 h. The release of dox from the DTT-treated hydrogel was clearly visible upon the color change of the hydrogel from deep red to almost transparent, which is shown in Fig. 8. The release of dox from the xerogel involves the simultaneous absorption of water/buffer and desorption of dox via a swelling controlled diffusion mechanism. The mechanism of dox release from the PAA-5cys + 5jeff was determined by fitting the data to the Ritger–Peppas equation:27where Mt/M∞ denotes the fractional release of the drug from the hydrogel, k denotes the proportionality constant, n is the release exponent and t is time. The release exponent was calculated from the slope of natural logarithmic plot of fractional release (initial linear region of the 60% release) versus time. Fig. 9 shows the fractional release of dox from PAA-5cys + 5jeff hydrogel with respect to time in the absence and presence of DTT. It is observed that the dox release from the hydrogel with DTT follows Fickian behavior (n < 0.5) and without DTT shows non-Fickian behavior (n > 0.5).
 |
| | Fig. 7 Cumulative release of dox with time from PAA-5cys + 5jeff in presence and absence of DTT and (a) structure of doxorubicin HCl. | |
 |
| | Fig. 8 PAA-5cys + 5jeff loaded with dox (a) dried in oven, (b) swollen in PBS (pH 7.4, 0.01 M) without DTT and (c) swollen in PBS (pH 7.4, 0.01 M) with DTT. | |
 |
| | Fig. 9 Fractional release of dox form PAA-5cys + 5jeff in presence and absence of DTT. | |
3.9 Microstructural characterization
Scanning electron microscopy was performed on dried AgNPs incubated gels in order to observe the change in morphology of the gels (see ESI, Fig. 5S†). The SEM image clearly indicates the porous structure in PAA-5cys + 5jeff hydrogels with AgNPs present on the surface. To further confirm the formation of AgNPs, Transmission Electron Microscopy (TEM) was performed by collecting the supernatant from the gel.
From Fig. 10, it is clear that the AgNPs were successfully formed inside the hydrogel matrix with an average size of 10 nm. The AgNPs were spherical in shape with a face centered cubic (fcc) structure, clearly observed as diffraction rings. Further confirmation of the presence of AgNPs in the hydrogel was obtained using energy-dispersive X-ray analysis (EDAX). Optical absorption peak at approximately 3 keV due to surface plasmon resonance confirms the presence of Ag in the hydrogel (Fig. 11). Table 4 shows the percentage of each element present in the hydrogel as obtained from EDAX.
 |
| | Fig. 10 TEM image of AgNPs in PAA-5cys + 5jeff. | |
 |
| | Fig. 11 EDAX image of Ag embedded in PAA-5cys + 5jeff hydrogel. | |
Table 4 Weight% of each element by EDAX
| Element |
Ag embedded in PAA-5cys + 5jeff hydrogel |
| C (K) |
63.44 |
| O (K) |
3.83 |
| S (K) |
4.54 |
| Cu (K) |
22.30 |
| Ag (K) |
5.86 |
3.10 Release of AgNPs from double-cross-linked PAA hydrogels
We also studied the release of AgNPs from the double-cross cross-linked PAA hydrogels by selectively cleaving the cystamine bonds using DTT. The release of AgNPs in the DTT-treated gels is clearly visible in Fig. 12 where the hydrogel that has released AgNPs becomes transparent. Fig. 13 shows the cumulative release of AgNPs from the gel as a function of time in the presence of DTT. It is observed that the hydrogel in the absence of DTT shows 5% AgNPs release in 7 days, whereas in the presence of DTT, a faster release was observed (48%). This slow release of nanoparticles from hydrogels could be explained by strong coordination bonds between Ag and –COONa groups of the gels. On addition of DTT, there is an increase in gel swelling that helps in the release of nanoparticles. Total AgNPs present inside the gel were calculated by probe sonicating the gel for 10 min and calculating the UV absorbance at 400 nm.
 |
| | Fig. 12 PAA-5cys + 5jeff loaded with (a) AgNPs (without DTT) and (b) AgNPs (with DTT). | |
 |
| | Fig. 13 Cumulative release of AgNPs with time from PAA-5cys + 5jeff in presence of DTT. | |
4. Conclusions
In conclusion, we successfully synthesized double-cross-linked PAA hydrogels simultaneously using cross-linking agents cystamine and jeffamine. The two diamines were coupled to PAA using the EDC coupling agent. All hydrogels were characterized in terms of structure, morphology, swelling and mechanical strength. The hydrogels obtained showed good mechanical strength and exhibited strong dependence on the degree of cross-linking and extent of swelling. Jeffamine showed more efficient cross-linking compared to cystamine. The selective breaking of disulphide linkage in the cystamine was realized using DTT, which resulted in reducing the degree of cross-linking and increasing hydrogel swelling. This phenomenon of in situ breaking of one cross-linking and increasing the swelling ratio could be used in swelling-controlled drug delivery systems. Selective breaking of cross-links on the swelling-controlled release of the anticancer drug, doxorubicin, was demonstrated. Further, in order to incorporate antimicrobial properties to the hydrogels, we successfully prepared AgNPs inside the gels. The gels could be used for controlled release of AgNPs over a period of time by selective cleavage of cystamine bonds. These double-cross-linked hydrogels show great promise in drug delivery and tissue engineering applications.
Acknowledgements
Financial support for this work was provided by the Council of Scientific and Industrial Research (CSIR), New Delhi, via grant CSC0134 (NT and MVB) and a senior research fellowship (NT).
Notes and references
- S. M. H. Bukhari, S. Khan, M. Rehanullah and N. M. Ranjha, Int. J. Polym. Sci., 2015, 15 Search PubMed.
- A. Chang, Iran. Polym. J., 2015, 24, 161–169 CrossRef CAS.
- Z. A. Hamid, A. Blencowe, B. Ozcelik, J. A. Palmer, G. W. Stevens, K. M. Abberton, W. A. Morrison, A. J. Penington and G. G. Qiao, Biomaterials, 2010, 31, 6454–6467 CrossRef CAS PubMed.
- S.-Y. Choh, D. Cross and C. Wang, Biomacromolecules, 2011, 12, 1126–1136 CrossRef CAS PubMed.
- J. Choi, H. J. Kung, C. E. Macias and O. K. Muratoglu, J. Biomed. Mater. Res., Part B, 2012, 100, 524–532 CrossRef PubMed.
- S. S. Halacheva, T. J. Freemont and B. R. Saunders, J. Mater. Chem. B, 2013, 1, 4065 RSC.
- X. Zhang, S. Malhotra, M. Molina and R. Haag, Chem. Soc. Rev., 2015, 44, 1948–1973 RSC.
- Z. M. Rzaev, S. Dincer and E. Pişkin, Prog. Polym. Sci., 2007, 32, 534–595 CrossRef CAS.
- D. Das, P. Ghosh, S. Dhara, A. B. Panda and S. Pal, ACS Appl. Mater. Interfaces, 2015, 7, 4791–4803 CAS.
- X. Gao, C. He, C. Xiao, X. Zhuang and X. Chen, Polymer, 2013, 54, 1786–1793 CrossRef CAS.
- Q.-B. Wei, F. Fu, Y.-Q. Zhang and L. Tang, J. Polym. Res., 2015, 22, 1–8 CrossRef CAS.
- H. Hosseinzadeh, J. Chem. Sci., 2010, 122, 651–659 CrossRef CAS.
- I. Manavi-Tehrani, M. Rabiee, M. Parviz, M. R. Tahriri and Z. Fahimi, Macromol. Symp., 2010, 296, 457–465 CrossRef CAS.
- J. Dobson, Drug Dev. Res., 2006, 67, 55–60 CrossRef CAS.
- T. Shiga, Y. Hirose, A. Okada and T. Kurauchi, J. Appl. Polym. Sci., 1992, 44, 249–253 CrossRef.
- J. Zhang, F. Yang, H. Shen and D. Wu, ACS Macro Lett., 2012, 1, 1295–1299 CrossRef CAS.
- X. Z. Shu, Y. Liu, Y. Luo, M. C. Roberts and G. D. Prestwich, Biomacromolecules, 2002, 3, 1304–1311 CrossRef CAS PubMed.
- A. N. Zelikin, Q. Li and F. Caruso, Chem. Mater., 2008, 20, 2655–2661 CrossRef CAS.
- M. Zrinyi, T. Gyenes, D. Juriga and J.-H. Kim, Acta Biomater., 2013, 9, 5122–5131 CrossRef CAS PubMed.
- B. Gyarmati, Á. Némethy and A. Szilágyi, RSC Adv., 2014, 4, 8764 RSC.
- M. Zhong, Y. T. Liu, X. Y. Liu, F. K. Shi, L. Q. Zhang, M. F. Zhu and X. M. Xie, Soft Matter, 2016, 12, 5420–5428 RSC.
- M. J. Kutyła, M. W. Boehm, J. R. Stokes, P. N. Shaw, N. M. Davies, R. P. McGeary, J. Tuke and B. P. Ross, AAPS PharmSciTech, 2013, 14, 301–311 CrossRef PubMed.
- T. Hussain, M. Ansari, N. M. Ranjha, I. U. Khan and Y. Shahzad, Sci. World J., 2013, 340737 Search PubMed.
- M. V. Patwadkar, C. S. Gopinath and M. V. Badiger, RSC Adv., 2015, 5, 7567–7574 RSC.
- X. Y. Jianbo Liu, K. Wang, Q. Wang, H. Ji, C. Wu, J. Li, X. He, J. Tang and J. Huang, J. Mater. Chem. B, 2012, 22, 495–501 RSC.
- Z. S. Liu, S. Swaddiwudhipong, F. S. Cui, W. Hong, Z. Suo and Y. W. Zhang, Int. J. Appl. Mech., 2011, 03, 235–257 CrossRef.
- P. L. Ritger and N. A. Peppas, J. Controlled Release, 1987, 5, 37–42 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of hydrogels, UV spectra of AgNPs embedded in hydrogel, Fickian curve and SEM of AgNPs embedded hydrogel. See DOI: 10.1039/c6ra19443e |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), cystamine di-hydrochloride, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), dithiothreitol (DTT) were obtained from Sigma-Aldrich, USA. Jeffamine (MW = 2000) was obtained from Texaco Chemical Company, USA. Doxorubicin HCl was obtained from Prolab Marketing Pvt. Ltd., New Delhi. Silver nitrate (AgNO3) was procured from S.D. Fine Chemicals Ltd. (India) and sodium borohydride (NaBH4) was purchased from Merck (India). All chemicals were of analytical grade and used as received.
000), cystamine di-hydrochloride, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl), dithiothreitol (DTT) were obtained from Sigma-Aldrich, USA. Jeffamine (MW = 2000) was obtained from Texaco Chemical Company, USA. Doxorubicin HCl was obtained from Prolab Marketing Pvt. Ltd., New Delhi. Silver nitrate (AgNO3) was procured from S.D. Fine Chemicals Ltd. (India) and sodium borohydride (NaBH4) was purchased from Merck (India). All chemicals were of analytical grade and used as received.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) jeffamine) and EDC coupling agents were placed in a cup and bob. The parameters, storage modulus (G′) and loss modulus (G″) were measured as a function of time at 25 °C. The percentage strain was 0.15% and the frequency was 10 Hz throughout the experiment. The time at which the crossover between G′ and G″ occurs was recorded as the time of gelation.
jeffamine) and EDC coupling agents were placed in a cup and bob. The parameters, storage modulus (G′) and loss modulus (G″) were measured as a function of time at 25 °C. The percentage strain was 0.15% and the frequency was 10 Hz throughout the experiment. The time at which the crossover between G′ and G″ occurs was recorded as the time of gelation.

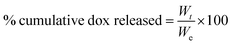

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively. These two peaks in the hydrogels indicate the formation of amide linkages between PAA and cystamine/jeffamine by EDC coupling reaction. On addition of DTT, in PAA-5cys + 5jeff hydrogel, a sharp peak appears at 2874 cm−1. This peak is attributed to the formation of –SH groups by successful breaking of S–S groups selectively in PAA hydrogel in the presence of DTT25 as shown in Fig. 2.
O groups, respectively. These two peaks in the hydrogels indicate the formation of amide linkages between PAA and cystamine/jeffamine by EDC coupling reaction. On addition of DTT, in PAA-5cys + 5jeff hydrogel, a sharp peak appears at 2874 cm−1. This peak is attributed to the formation of –SH groups by successful breaking of S–S groups selectively in PAA hydrogel in the presence of DTT25 as shown in Fig. 2.














